Feature | The 20th Anniversary of TAVR: The View From Our Front Row Seats!
We first became acquainted in 2004 when we met at the Schiphol Airport Hotel in Amsterdam during the formation of the Steering Committee for the SYNTAX trial. Little did we know then how inextricably bound our careers would become! We spent the next seven years as professional colleagues and casual acquaintances at medical meetings, often as debate opponents discussing the merits of drug-eluting stents or CABG. As if not challenging enough, we on occasion took the opponent's position using their slides. This was not always eagerly embraced by the conference organizers, but the information we presented was always correct and based on the literature but tempered with perhaps some shading of interpretation. This also formed the basis of what has become the concept of the Heart Team.
Building Relationships
All of this evolved significantly by serendipitous good fortune in February 2012! We had become the respective presidents of the ACC (Holmes) and the Society of Thoracic Surgeons (STS; Mack). In our roles, we each received an invitation to meet in Washington, DC, with Bram D. Zuckerman, MD, FACC, director of the Office of Cardiovascular Devices at the U.S. Food and Drug Administration (FDA). What quickly ensued was an "on the job" primer course in health care policy for both of us. Aided by the expertise of the government relations/advocacy staff of our two societies, we quickly became schooled in such arcane policy terms and acronyms as advisory panels, safe and effective, reasonable and necessary, postmarket device surveillance, and premarket approval and National Coverage Determination (NCD).
At the initial meeting, we were informed the FDA would be holding an Advisory Committee (termed Panel) meeting in July 2012 to consider regulatory approval of transcatheter aortic valve replacement (TAVR). We were asked if the professional societies would be interested and willing to assist the FDA and the device sponsor, Edwards Lifesciences, in monitoring the "real world" performance of TAVR if the device was approved by the FDA. Thus began a collaborative relationship between the two professional societies, the FDA, the Centers for Medicare and Medicaid Services (CMS) and the device sponsors, initially Edwards Lifesciences and subsequently Medtronic, that led to the "rational dispersion," a term coined by Ralph G. Brindis, MD, MPH, MACC, of TAVR into the US.
The Making of a Registry
We both testified at the FDA Panel on behalf of our professional societies and offered the expertise and experience of our database leaders and staff to help build a new database to monitor TAVR device performance – thus creating the STS/ACC TVT Registry.
The STS/ACC TVT registry has now captured the outcomes on >400,000 patients undergoing TAVR procedures in the U.S., representing virtually every patient treated in nongovernment institutions and outside of clinical trials. This is a unique achievement and a defining example of what can be achieved by multiple stakeholders working together.
Lessons Learned
Along with the safe introduction and rapid adoption of TAVR into the U.S. and a paradigm shift in the management of aortic stenosis, other important developments have ensued, including ascertainment based on robust evidence that the outcomes with TAVR in "real world" practice approximate those obtained in highly selective and regulated clinical trials.
Additionally, there has been the development of a new health care policy paradigm through the coordination between the FDA and CMS. When TAVR was initially approved by the FDA in prohibitive surgical risk patients, CMS issued an "evergreen" NCD providing reimbursement for TAVR for any "FDA approved indication." This policy not only provided national reimbursement for TAVR procedures but also obviated the need for reopening a coverage determination with each subsequent label expansion.
Credit for the development of this seamless coverage policy goes to Louis Jacques, MD, and Tamara Syrek-Jensen, JD, directors of the Coverage and Analysis Group who worked closely and collaboratively with Jeffrey E. Shuren, MD, JD, and Zuckerman at the FDA.
Other key takeaways include:
- Use of the STS/ACC TVT Registry as a new platform for postmarket device surveillance and nested registries for label expansion. Key to the success of this platform was the agreement of Edwards Lifesciences to make the "leap of faith" to support and help build the TVT Registry given the uncertainties of this new paradigm of introducing an innovative medical device into clinical practice. The contributions of Larry L. Wood, corporate vice president of Edwards Lifesciences, were crucial in making the STS/ACC TVT Registry a success at the earliest stages.
- Development of the STS/ACC TVT Registry Stakeholders Advisory Group, which includes clinicians, regulators, payers, industry device sponsors, patient advocates, the National Institutes of Health, hospitals and health care quality experts, all working collaboratively to ensure the best medical care to the patients in the U.S.
- Development and adoption of the "Heart Team." The multidisciplinary team-based concept of care is, of course, not a new one, being initially described at the Mayo Clinic in the 1950s for cardiac care and widely adopted in the transplant and oncology fields. However, the prominence of the role of the heart team in TAVR arguably may be one of the more consequential benefits of the introduction of TAVR.
Celebrate 20 Years of TAVR at ACC.22
Join these anniversary events at ACC.22 on the Heart 2 Heart Stage, in the Lounge & Learn Pavilion, Hall D.
Sunday, April 3, 10:30 – 11 a.m.
ACC President's Picks Interview With Dr. Alain Cribier
Join ACC President Dipti Itchhaporia, MD, FACC, for a discussion with Cribier on his work and the lessons learned to date.
Monday, April 4, 7:30 – 8:15 a.m.
Disruptive Innovation: TAVR 20 Years Later
Join Michael J. Mack, MD, MACC, and other TAVR pioneers for a conversation about ways disruptive innovation can deliver far-reaching transformational change in care delivery.
Modern-Day Teamwork

The role of the modern-day Heart Team was critical in the conduct of the SYNTAX trial, in which the interventional cardiologists and cardiac surgeons had to agree that a patient with coronary artery disease was equally revascularizable by percutaneous and surgical approaches. This collaborative team-based approach to care was even more seminal in the conduct of the pivotal trials in TAVR.
The Heart Team approach to TAVR embraced collaboration from the original trial design to active procedure performance together to rational dispersion of the new procedure and generation of real-world evidence.
Based on the success of the Heart Team in the regulatory trials, both FDA approval regulations and CMS reimbursement conditions require Heart Team-based care. The team approach has subsequently been employed in mitral and tricuspid valve trials and has had a positive impact on everyday clinical practice with interdisciplinary care becoming the norm in many institutions. In the field of left atrial appendage occlusion, reimbursement is specifically predicated on documentation of a team-based evaluation for patient selection.
The size and composition of the Heart Team has changed based on the disease being treated, and now includes imaging specialists, general cardiologists, advanced practice providers, administrators and social workers. It has spanned a number of other individuals and diseases, such as team-based care for stroke, peripheral arterial disease, pulmonary embolism response team, thoracic aortic disease, extracorporeal membrane oxygenation and high-risk coronary artery disease.
Celebrating Success
We are now celebrating the 20th anniversary of the first-in-human TAVR procedure. We have witnessed a monumental shift in the management of a disease in a relatively short period of time. We also should celebrate all the collateral benefits.
A number of individuals collectively need to be recognized for their consequential roles in these events, including the early investors and industry sponsors who took the risk and made the bold leap into transcatheter therapy; our patients and their families who placed their faith and confidence in their caregivers and this new therapy; the intrepid clinicians and trialists who lived on the edge and worked so diligently to benefit their patients.
Finally, and maybe most importantly, the work of our professional colleagues at the FDA and CMS, who work with humility and grace and without recognition yet strive everyday to bring innovative treatments safely and expeditiously to the U.S. citizens they serve needs to be highlighted.
The U.S. was the 43rd country to approve TAVR. Led by Shuren at the FDA, the introduction of TAVR has been used to bring early feasibility trials to the U.S. and to develop a collaborative community with the clinical community, professional societies, industry and patient advocates to bring innovation to the U.S. There are many other individuals who deserve credit and recognition; you know who you are, and you should be justifiably proud of your contributions. It really does take a team.
By fate and serendipity, the two of us have been fortunate to have front row seats to witness these changes over the past two decades. We are privileged to have rubbed elbows with many of the diverse players in this well-orchestrated evolution (arguably revolution!). We applaud their performance and appreciate the opportunity we have had to experience this masterful performance from our seats in the front row.


This article was authored by David R. Holmes Jr, MD, MACC, an interventional cardiologist at Mayo Clinic in Rochester, MN, and Michael J. Mack, MD, MACC, a cardiothoracic surgery at Baylor Scott & White Health in Plano, TX.
TAVR at 20: A Model For the Future?

"The TAVR odyssey has been driven by the rapid enhancement of technology, a true commitment to collaboration across stakeholders, and a trust and reliance in the scientific process and the constant push to refine and improve – all with the goal of doing what is best for patients," writes Dipti Itchhaporia, MD, FACC, in a recent Leadership Page in JACC.
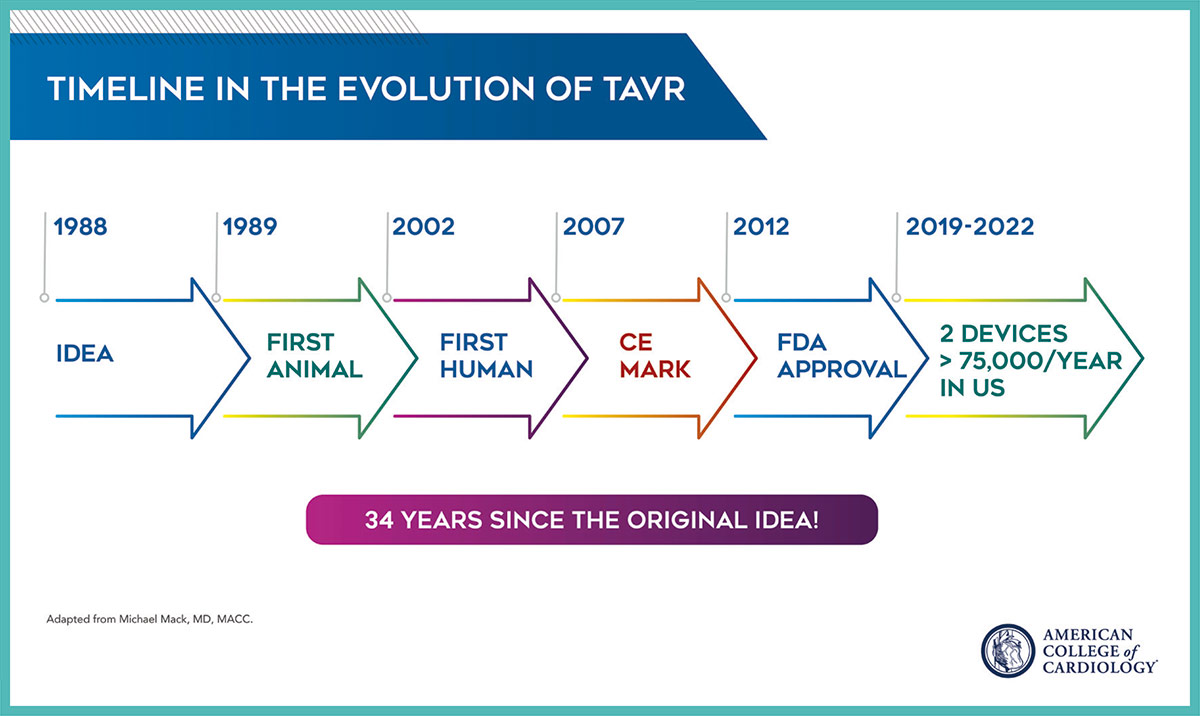
TAVR did not arrive on the scene overnight. The road to TAVR began in the 1980s and 1990s with a number of inspiring individuals, including Presidential Citation recipient Alain Cribier, MD, FACC, contributing to the scientific endeavor of developing, testing and building on a hypothesis and working with engineers and others to develop a workable device. And then many more, including Holmes, Mack, Martin B. Leon, MD, MACC, and others, contributing to the randomized clinical trials that led to the approval of TAVR in the U.S in 2012 and ultimately where we are today. Even now, the TAVR journey is not over, with more long-term research and device durability studies in the works.
Looking forward, Itchhaporia writes: "My hope is that we – as a profession and a College – can use the story of TAVR to help us write new stories. … Disruptive innovation is something we should run toward, not away from. Previously, inoperable patients with severe AS had no options. Today they do. That is transformation at its finest."
Clinical Topics: Cardiac Surgery, Cardiovascular Care Team, Invasive Cardiovascular Angiography and Intervention, Valvular Heart Disease, Atherosclerotic Disease (CAD/PAD), Aortic Surgery, Cardiac Surgery and VHD, Interventions and Coronary Artery Disease, Interventions and Structural Heart Disease, Interventions and Vascular Medicine
Keywords: ACC Publications, Cardiology Magazine, Advisory Committees, Aortic Diseases, Aortic Valve Stenosis, Cardiologists, Caregivers, Centers for Medicare and Medicaid Services, U.S., Coronary Artery Bypass, Coronary Artery Disease, Delivery of Health Care, Documentation, Drug-Eluting Stents, Extracorporeal Membrane Oxygenation, Feasibility Studies, Friends, Health Policy, Hospitals, Medicaid, Medicare, National Institutes of Health (U.S.), Noncommunicable Diseases, Patient Advocacy, Patient Care Team, Patient Selection, Peripheral Arterial Disease, Pulmonary Embolism, Registries, Social Workers, Stroke, Surgeons, Transcatheter Aortic Valve Replacement, Tricuspid Valve, United States Food and Drug Administration, ACC22, ACC Annual Scientific Session, National Cardiovascular Data Registries, STS/ACC TVT Registry, ACC Scientific Session Newspaper, Newspaper Article
< Back to Listings