Anemia and Heart Failure: Guidance for Clinicians and Trialists
Quick Takes
- Provides a re-evaluation of how best to define and measure true anemia in heart failure patients.
- Examines some of the pitfalls and fallacies leading to under recognition and treatment of anemia, despite expert recommendations.
- Provides thoughts on ways forward towards a more cohesive and unified approach to allow better patient outcomes.
Anemia, the reduction in the red blood cell volume, from whatever cause, can be very impactful on the progression of heart failure (HF). It triggers reduction in oxygen carrying and delivery capacity, and thus counters the key function of the circulation. Anemia is common in both general internal medicine populations1 and in patients with cardiovascular disease.2 Specifically for patients with HF, the incidence in hospitalized patients varies widely between 10% and up to 50%.3 Strong relationships exist between anemia and demographics and outcomes including, older age and female sex, diabetes, chronic kidney disease, worse functional status, lower life quality.4-10 An increased risk of death has been associated with anemia in heart failure patients.
Expert guidance organizations continue to ask that we seek and treat anemia in patients with HF.11 In a recent American College of Cardiology (ACC) Expert Consensus Decision Pathway Document focusing on patients hospitalized for HF,12 one of 10 key points includes anemia assessment and treatment throughout the hospitalization period.
The earliest treatment approaches13-14 suggested that erythrocyte stimulating agents (ESAs) and intravenous iron were an approach that benefited patients. However, the enthusiasm for using ESAs waned after a large, randomized, clinical trial showed a lack of a mortality benefit and increased serious adverse events in patients treated with an ESA.15 Applying a target peripheral hemoglobin of ≥13g/dl, the risk of embolic and thrombotic events was significantly higher in a group of patients receiving darbepoetin-alfa than in patients receiving placebo.
Finally, it is clear in HF patients, as in many other chronic disease states, there is a common overlapping syndrome of iron deficiency. We do know from recent trials of IV iron supplementation (ferric carboxymaltose) in patients identified during an acute HF hospitalization, by serum iron markers, there was reduced risk of recurrent hospitalization yet no impact on risk of cardiovascular death.16 Further, patients included and treated had an average baseline hemoglobin of 12.3 g/dL and 52% were "anemic" by their criteria.
Presence of iron deficiency, independent of anemia, is related to impaired oxidative metabolism, poor cellular energetics, impaired immune mechanisms, and decrease oxygen storage in myoglobin and reducing tissue oxidative capacity.3 In the FAIR-HF trial, a small increase of hemoglobin was seen in anemic patients while none was present in non-anemic patients. Still, patients with and without anemia benefited with improvements of symptoms, exercise capacity and six-minute walk distance.17
Within that simplified frame, it is clear that we need to move ahead to better understand what anemia is and how it is best measured. Here, we believe are some of the questions we need to be asking:
- Is anemia an unmet medical need (detection and treatment)?
- What should be the definition of anemia?
- How to screen and test?
- What outcomes should we expect?
- What are the ways forward?
Unmet Medical Need
The wide ranges of the estimated presence of anemia in heart failure patients strongly suggests that anemia prevalence in patients with Stage C and D heart failure is under detected and undertreated. However, the ability to identify and enroll patients in current and prior clinical trials with anemia or iron deficiency syndromes suggest a huge repository of at risk yet untreated patients. Our opinion is that several aspects of heart failure mythology allow this misstep to proceed, including that perception that a low peripheral hematocrit generally reflects the "volume" overload status of heart failure patients. When direct measurement of intravascular volume and red blood cell volumes have been measured upon admission to an acute care hospital for treatment of presumed decompensated heart failure, only 37% were hypervolemic yet 62% had true anemia.18
Defining Anemia
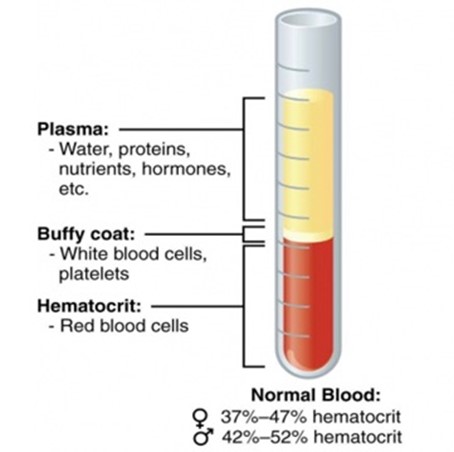
The complex catalog of mechanisms and forms of anemia have at its core a reduction in red blood cell volume. In 1929, Wintrobe used capillary tubes to display the relationships between intravascular plasma volume and red blood cell volume.19 Like all things in nature, there is a tightly controlled range of normal relationships of these two intravascular volume compartments, and variability is at the root of dysregulation, dysfunction and disease progression. The issue is that a peripherally drawn hematocrit reports a ratio without knowledge of the true plasma or red blood cell volumes. (Figure 1)
Figure 1
Noumi and colleagues compared differences between anemia prevalence using the peripheral hematocrit (World Health Organization [WHO] criteria) and direct measurement of red blood cell volume using blood volume analysis in a cohort of racially diverse patients with heart failure with preserved ejection fraction (HFpEF) (n=60).20 Using the peripheral hematocrit, "anemia" was present in 67%, whereas only 35% of the patients actually had a reduced red blood cell volume (true anemia). Further, the WHO criteria revealed no racial disparities, whereas the blood volume analysis method showed a two-fold higher prevalence among Hispanics compared to Whites or Blacks.
Screening and Testing
These words for us have different meanings and connotations and are part of this issue. Screening can mean looking for illness in an otherwise healthy population. Indeed, this is a goal of large international organizations who are looking to find anemia in populations in order to improve overall health. Alternatively, screening can also mean looking for an abnormality in populations in whom the abnormality significantly impacts their underlying health issues and the risks of progression. In patients with heart failure, we are asked to test for anemia precisely for those reasons. Lastly, testing means seeking targeted results based on clinical situations, signs and symptoms of existing or emerging disease states. For the first, field peripheral hematocrit levels may be suitable. For the last two scenarios, where the results become part of the patient's disease trajectory and can dramatically inform treatments and outcomes, greater precision is required, and every attempt should be made to measure and know total blood volume; for patients with heart failure, knowing their total blood volume phenotype seems beneficial.
Outcomes to Expect
Restoration of a normalized red blood cell volume should provide a step away from deranged physiology and a step towards recovery. Therefore, we should expect that whatever therapies are used, discovered, re-discovered or combined, should provide patient level improvement in functional status, life quality, slow disease progression and significantly impact outcome metrics that define success for HF patients, including days alive and out of the hospital, improved functional status, life quality and survival. For society, this should impact the global cost of care, including admissions and readmissions, especially for chronic disease states for which anemia is such a common and important overlay.
Ways Forward
The concept that we need to redefine anemia measurement may sound unusual, at first. However, connecting the current gaps in awareness of anemia prevalence in heart failure patients, the impact of current anemia diagnostics, and the potential for therapeutics to be more precisely targeted to improve safety and outcomes, it becomes clearer to us that there is some missing link—and it just may be how we think about what anemia represents and the priority we place on its measurement, management, and treatment.
Summary Thoughts
Figure 2
The topic of anemia in HF is one that is complicated by imprecision in definitions, diagnostics, and therapeutic targets. Therein lies an opportunity to re-prioritize anemia as a defined disease that is related to a deficiency in red blood cell volume. There is a clear overlap with the iron deficiency syndrome. Yet for now, they remain important but separate treatment targets.
References
- Randi ML, Bertozzi I, Santarossa C, et al. Prevalence and causes of anemia in hospitalized patients: impact on diseases outcome J Clin Med 2020;9:950.
- Kansagara D, Dyer E, Englander H, Freeman M, Kagen D. Treatment of anemia in patients with heart disease: a systematic review (va.gov). 2011. Available at: https://www.hsrd.research.va.gov/publications/esp/anemia-REPORT.pdf. Accessed 05/15/2021.
- Anand IS, Gupta P. Anemia and iron deficiency in heart failure current concepts and emerging therapies. Circulation 2018;138:80–98.
- Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002;39:1780–86.
- Mozaffarian D, Nye R, Levy WC. Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE). J Am Coll Cardiol 2003;41:1933–39.
- Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation 2003;107:223–25.
- Anand I, McMurray JJV, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation 2004;110:149–54.
- He SW, Wang LX. The impact of anemia on the prognosis of chronic heart failure: a meta-analysis and systemic review. Congest Heart Fail 2009;15:123–30.
- Lindenfeld J. Prevalence of anemia and effects on mortality in patients with heart failure. Am Heart J 2005;149:391–401.
- Maggioni AP, Opasich C, Anand I, et al. Anemia in patients with heart failure: prevalence and prognostic role in a controlled trial and in clinical practice. J Card Fail 2005;11:91–98.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-e239.
- Maddox TM, Januzzi JL Jr, Allen LA, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021;77:772-810.
- Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function, functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol 2000;35:1737– 44.
- Silverberg, DS, Wexler, D, Sheps, D, et al. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol 2001;37:1775-80.
- Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure (RED-HF). N Engl J Med 2013;368:1210-19.
- Ponikowski P, Kirwan BA, Anker SD, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomized, controlled trial. Lancet 2020;396:1895-1904.
- Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361:2436–48.
- Strobeck JE, Feldschuh J, Miller WL. Heart failure outcomes with volume-guided management. JACC Heart Fail 2018;6:940-48.
- Wintrobe MM. A simple and accurate hematocrit. J Lab Clin Med 1929;15:287-9.
- Noumi B, Teruya S, Solomon S, Helmke S, Maurer MS. Blood volume measurements in patients with heart failure and a preserved ejection fraction (HFPEF): implications for diagnosing anemia. Congest Heart Fail 2011;17:14–18.
Clinical Topics: Heart Failure and Cardiomyopathies, Prevention, Nonstatins, Acute Heart Failure, Diet, Stress
Keywords: Heart Failure, Anemia, Myoglobin, Plasma Volume, Hematocrit, Quality of Life, Benchmarking, Patient Readmission, African Americans, Anemia, Iron-Deficiency, Cardiovascular Diseases, Exercise Tolerance, Goals, Stroke Volume, Hemoglobins, Chronic Disease, Hispanic Americans, Iron, Diabetes Mellitus, Disease Progression, Erythrocytes, Hospitals, Renal Insufficiency, Chronic, Plasma, Internal Medicine, Phenotype, Dietary Supplements, Oxidative Stress, World Health Organization, Oxygen
< Back to Listings