The Role of Primary ASCVD Prevention Among Patients Newly Diagnosed With Cancer
Quick Takes
- The development of cardiovascular disease and some cancers share common risk factors, prompting an interest in primary atherosclerotic cardiovascular disease (ASCVD) risk assessment and management.
- Cardiovascular risk factors, plasma, and imaging biomarkers can help identify high-risk patients.
Primary prevention of ASCVD in patients with cancer requires a multifactorial approach that takes into account an understanding of clonal hematopoiesis of indeterminate potential (CHIP), early identification of high-risk patients, and employment of cardiac biomarkers for identification of subclinical disease to prevent subsequent damage and modulate risk.1
Identification of High-Risk Patients
In the absence of guidelines specifically directed at this unique population, risk assessment should begin with calculation of short- and long-term ASCVD risk as suggested by current American Heart Association and American College of Cardiology multi-society guidelines, acknowledging that risk may be overestimated or underestimated.1 Additional consideration should be given to established risk factors for cardiovascular diseases including physical inactivity, poor nutrition, obesity, smoking, alcohol, hypertension, hyperlipidemia, and diabetes mellitus, many of which are discovered at the time of cancer diagnosis.1
Physical Inactivity
Physical inactivity remains one of the most significant modifiable risk factors for ASCVD worldwide. It has been shown that physical inactivity is responsible for approximately 12% of the global myocardial infarction burden, and the consequent disability-adjusted life years have grown significantly worldwide (from around 8.61 million in 1990 to 15.7 million in 2019).2 Thus, encouraging physical activity and incorporating cardio-oncology rehabilitation,3 which may reduce cardiovascular disease burden overall, can potentially reduce the risk of development of ASCVD in patients newly diagnosed with cancer. Specifically, the Physical Activity Guidelines for Americans recommends that adults do at least 150-300 minutes a week of moderate-intensity aerobic exercise to 150 minutes a week of vigorous-intensity aerobic exercise for maximal health benefit.4
Nutrition
Dietary habits constitute an essential aspect of both cardiac and oncological primary prevention. In fact, it has been shown that patients randomized to receive a balanced diet such as the Mediterranean diet had a 30% reduction in the risk of stroke, myocardial infarction, or cardiovascular death compared to controls.5 More specifically, the American Heart Association dietary guidelines focused on overall healthy eating patterns, maintaining an appropriate body weight, maintaining a desirable cholesterol profile, and maintaining a desirable blood pressure profile. This can be achieved by following guideline-directed dietary interventions including, but not limited to, including fruits, vegetables, grains, low-fat dairy, fish, and lean meats in the diet; maintaining healthy weight and matching energy intake to energy needs; and limiting foods high in saturated fats, cholesterol, and salt and alcohol.6
Obesity
An elevated body mass index is independently associated with all-cause, cancer-specific, and cardiovascular mortality among patients with cancer, especially those with colorectal cancer.1 Other studies have shown a correlation between waist circumference and increased cardiovascular disease in breast cancer survivors.7 Patients newly diagnosed with cancer are likely expected to derive benefit from weight loss as well, and maintenance of a normal body mass index in patients newly diagnosed with cancer is likely to lower the risk of incident cardiovascular disease.8
Smoking
Cigarette smoking is associated with an increase in all-cause mortality and cardiovascular disease in patients with newly diagnosed cancer and is an independent risk factor for the development of cancer and atherosclerosis.9 Identification of tobacco use patterns in patients newly diagnosed with cancer, and the provision of smoking cessation programs as available institutionally, may help prevent or reduce the development of cardiovascular diseases.9
Alcohol
Alcohol consumption in moderation has been shown in some studies to be cardio-protective.10 In excess, however, there is an overall detrimental effect and increased risk for the development of ASCVD.10 Also, alcohol itself has been associated with an increased risk of certain cancers, including oral and pharyngeal cancer, esophageal squamous cell carcinoma, colorectal cancer, laryngeal cancer, and breast cancer, all to varying degrees.9 Promoting alcohol cessation in patients newly diagnosed with cancer may help reduce the burden of cardiovascular disease in this patient population.11
Hypertension
Chemotherapeutic agents and medications used in the treatment of cancer may cause elevated blood pressure during the course of treatment.12 A variety of chemotherapeutic agents and cancer treatments can cause hypertension using different mechanisms (Table 1).12,13 Treatment with chemotherapy is an independent risk factor for hypertension due to direct effects of many agents on endothelial function, sympathetic activity, and renin-angiotensin system activity, as well as nephrotoxicity.13 In the absence of high-quality evidence, close monitoring, early identification of elevations in blood pressure, and management of hypertension should be balanced with considerations to polypharmacy, drug-drug interactions, risk of orthostatic hypotension, and metabolic derangements that can be encountered during chemotherapy treatment to reduce morbidity and mortality.13,14
Table 1: Chemotherapy and Cancer-Related Agents Associated With Hypertension13
| Anti-vascular endothelial growth factor therapy and tyrosine kinase inhibitors |
| Alkylating and alkyl-like agents |
| Cyclophosphamide |
| Ifosfamide |
| Cisplatin |
| Vinblastine |
| Gemcitabine |
| Radiation |
| Abdominal radiation |
| Head and neck radiation |
| Adjuvant therapies |
| Erythropoietin stimulating agents |
| Nonsteroidal anti-inflammatory drugs |
| Corticosteroids |
| Calcineurin inhibitors |
Hyperlipidemia
Chemotherapeutic agents also disturb lipid measurements during the course of cancer treatment. Studied mostly for breast cancer, it has been shown that certain agents such as doxorubicin and paclitaxel caused increases in levels of high-density lipoprotein cholesterol and low-density lipoprotein cholesterol during treatment.15 This is driven by various mechanisms, including reducing the expression of ABCA1 and apoaA1 with doxorubicin and increasing apoB and HMG-CoA reductase levels with both doxorubicin and paclitaxel.15 These effects, however, seem to be transient and have been shown to normalize 6-12 months after stopping chemotherapy.16 Screening lipid panels prior to starting chemotherapy and the initiation of statin therapy should follow available guidelines.17 It is worth noting that there are proposed frameworks for integrating ASCVD- and cancer-specific factors into decision-making for lipid-lowering agents that take into account the degree of ASCVD risk and cancer status.18
Diabetes Mellitus
Diabetes mellitus type 2 has been associated with higher rates of cardiovascular death, cancer-related death, and development of cancers than in patients without diabetes.19 The association of diabetes with cancer is cancer-site specific. The strongest relationships have been associated with liver and pancreatic cancer. Risk of endometrial cancer doubles in women with diabetes; risks of breast, colorectal, bladder, non-Hodgkin's lymphoma, and bladder cancer are 20-40% higher in diabetic patients.20 The identification and treatment of patients with diabetes or pre-diabetes according to established guidelines may help prevent or reduce the development of cardiovascular disease in patients newly diagnosed with cancer.20 It should be noted that achieving optimal glycemic control during cancer treatment may be challenging because the use glucocorticoids and chemotherapeutic agents (that may cause nephrotoxicity or cardiotoxicity), for example, may complicate the ability to control blood glucose.21
Modulation of Chemotherapy
Several types of chemotherapy may cause an increased risk of ASCVD and major adverse cardiac events, whether directly or indirectly. These may include tyrosine kinase inhibitors, vascular endothelial growth factor inhibitors, antimetabolites, platinum-based therapies, immune checkpoint inhibitors, and some hormonal therapies.22,23 The mechanism by which they affect cardiac tissue or may increase ASCVD risk are summarized in Table 2.22,23 Closer monitoring of patients receiving these therapies and increased surveillance of cardiotoxic effects prior to and during the course of treatment is likely necessary.
Table 2: Chemotherapy and Associated Cardiac Effects22,23
| Chemotherapy | Cardiac Effects |
| Tyrosine kinase inhibitors | Coronary vascular events, QT prolongation, hypercholesterolemia, hypertriglyceridemia |
| Vascular endothelial growth factor inhibitors | Hypertension, venous and arterial thromboembolic events, cardiomyopathy |
| Antimetabolites | Myocardial ischemia, arrhythmia |
| Platinum | Myocardial ischemia, hypertension |
| Immune checkpoint inhibitors | Myocarditis |
| HER2 inhibitors | Congestive heart failure and reduced ejection fraction |
Modulation of Radiation Therapy
Although newer radiation techniques may not pose the same risk as older methods, radiation-induced coronary heart disease was considered the second overall most common cause of morbidity and mortality.24 This risk is even higher in the presence of other cardiovascular risk factors.24 There are a variety of techniques that can be used to modulate radiation burden, and they are detailed in Table 3.
Table 3: Radiation Therapy Techniques to Reduce Radiation Dose25
| Technique | Mechanism |
| Heart blocks | Radiation field design avoiding the heart |
| Breast board | Improves angle of treatment along the chest wall to spare the anterior heart |
| Prone positioning | Decreases volume of heart in the radiation field for 85% of patients |
| Deep inspiration breathing technique | Treatment delivered when least heart volume is in the radiation field after deep inspiration and hold |
| Accelerated partial breast irradiation | Radiation of partial breast in select patients with early-stage breast cancer |
| Hypofractionation | Alternative schedule regimen with no added cardiac toxicity |
| Fixed gantry intensity-modulated radiation therapy | Reduced cardiac dosing compared to rotational intensity-modulated radiation therapy |
| Proton use | Lower cardiac dose compared to photon therapy |
ASCVD Evaluation in Cancer-Specific Screening
Breast Cancer Screening
Breast arterial calcification, which can be assessed from mammograms, represents a potential risk stratification tool and surrogate marker of ASCVD.26 Indeed, multiple studies have suggested a strong association between breast arterial calcification and cardiovascular disease or coronary artery disease, independent of other known cardiovascular disease risk factors.26 This association is hypothesized to be due to breast arterial calcification being a marker for increased atherosclerosis in all vascular territories including the coronary tree. Thus, the presence of breast arterial calcification may trigger they initiation of primary prevention pharmacotherapy in the appropriate setting.27 This presents an opportunity to provide preventive interventions without any extra cost or radiation involved.
Lung Cancer Screening
Coronary artery calcium (CAC) score has been shown to be a strong predictor for the development of future cardiovascular events. Chest imaging by way of chest computed tomography (CT), including low-dose CT scans, is an essential tool for the diagnosis and staging of lung cancer and can identify coronary artery calcification.28 Multiple studies have suggested a benefit for calculating CAC score on all CT scans of the chest in patients with cancer and patients being screened for lung cancer in order to implement cardiovascular protective strategies to reduce the rate of cardiovascular events in the future.28 Prior studies have reported presence of CAC in up to 62% of patients being screened for lung cancer, with up to 98% of those qualifying for statin initiation.29 Also, thoracic artery calcium score detected on lung cancer screening CT scans of the chest were found to correlate with CAC score.30 Thus, patients receiving screening scans who are found to have extensive thoracic aorta calcifications can possibly benefit from the same preventive measures as those with elevated CAC scores.
Colon Cancer Screening
Abdominal aortic calcification is associated with known cardiovascular risk factors and is positively correlated with CAC.31 CT abdominal aortic calcification has been shown to be a strong predictor of future cardiovascular events, even outperforming the Framingham score in one study.32 The finding of abdominal aortic calcification on screening abdominal CT can therefore help identify patients at high risk of ASCVD.
CHIP
CHIP refers to somatic mutations in hematopoietic cells yielding mutated leukocytes that predispose an individual to an increased risk of ASCVD as well as hematological malignancies.33 The mechanism by which CHIP increases the risk for ASCVD is not entirely understood, but includes 1) causing ASCVD directly via mutations, 2) accompanying aging and contributing indirectly to risk of ASCVD, or 3) reflecting a common risk factor that increases risk of ASCVD.33 Experimental evidence in mice and in vitro suggests a direct causal relationship between CHIP and ASCVD.2 CHIP can be detected through DNA sequencing of peripheral blood, saliva, and tumor samples (through blood contamination).34 Examples include Jak2V617F and TET2 mutations.35,36 Jak2V617F mutations have been associated with atheromata that contain more neutrophils, have larger lipid cores, and demonstrate increased iron deposition in mice models, leading to more plaque formation and an increased risk for rupture.35 TET2-deficient cells were found to exhibit increased NLRP3 inflammasome-mediated interleukin-1 beta secretion in mice models, which was directly correlated with increased atherosclerotic plaque size.36 The recognition of CHIP strengthens the link between oncology and cardiovascular disease, more so at older age. Considering that CHIP may add to the list of common risk factors that predispose to ASCVD and cancer, its presence offers an opportunity to implement genotype-driven personalized primary prevention methodologies. Although this has been a growing field of interest, integration into current practice guidelines remains unavailable.33
Serum Biomarkers
Serum biomarkers, including high-sensitivity troponin and N-terminal pro-B-type natriuretic peptide, have been extensively studied for early detection of chemotherapy-related cardiotoxicity and resulting heart failure.37,38 However, their use as markers of ASCVD has not been validated and cannot yet be used to guide ASCVD prevention management.39
Conclusion
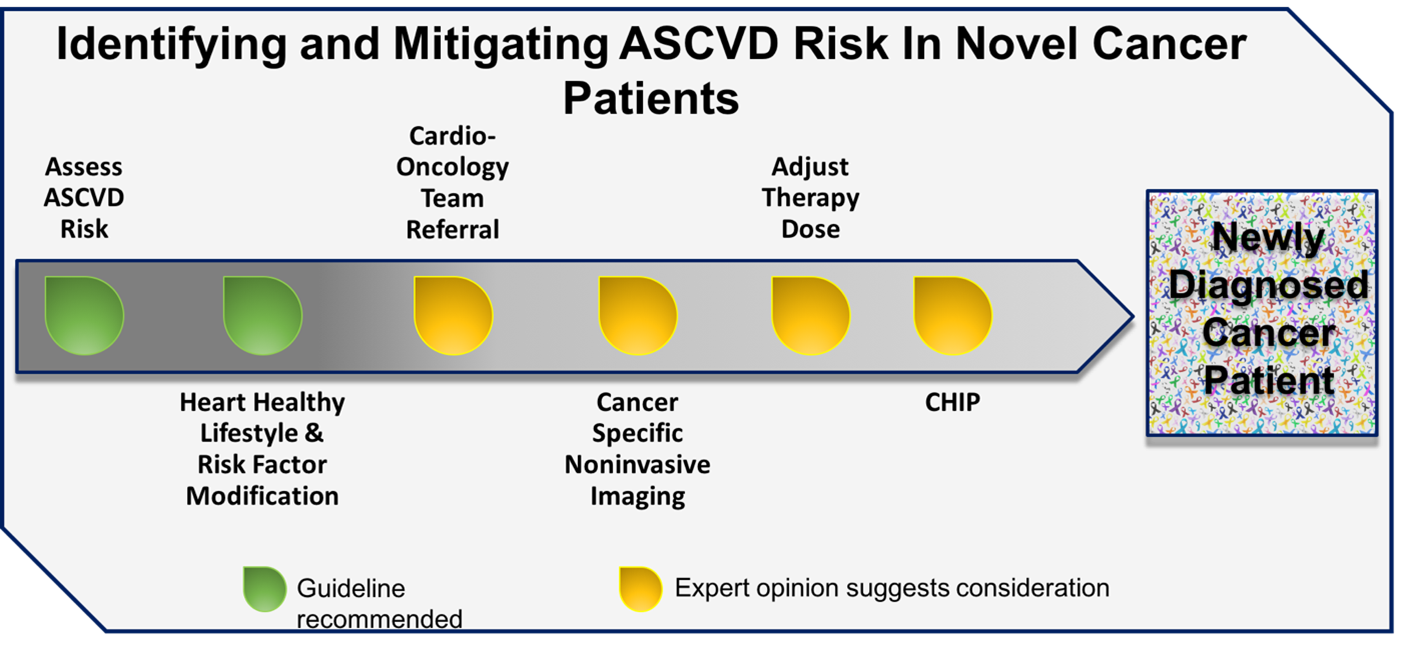
Patients newly diagnosed with cancer are often discovered to have cardiovascular risk factors, which inherently increase the risk of cardiotoxicity. Beyond traditional risk assessment methodologies, the presence of risk factors common to ASCVD and cancer may also identify those at heightened risk for the future development of ASCVD. Cancer-specific imaging, namely CT, allows for the identification of calcific vascular disease, which is known to be a strong predicator of future cardiovascular events. Timely institution of available primary prevention interventions can halt progression of disease and potentially reverse adverse remodeling resulting from the effects of cancer therapy (Figure 1). These efforts are required to mitigate the excess risk of cardiovascular disease in patients newly diagnosed with cancer.
Figure 1: Primary Prevention of ASCVD in Patients With Novel Cancer
References
- Bravo-Jaimes K, Marcellon R, Varanitskaya L, et al. Opportunities for improved cardiovascular disease prevention in oncology patients. Curr Opin Cardiol 2020;35:531-37.
- Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982-3021.
- Gilchrist SC, Barac A, Ades PA, et al. Cardio-Oncology Rehabilitation to Manage Cardiovascular Outcomes in Cancer Patients and Survivors: A Scientific Statement From the American Heart Association. Circulation 2019;139:e997-e1012.
- Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA 2018;320:2020-28.
- Toledo E, Salas-Salvadó J, Donat-Vargas C, et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern Med 2015;175:1752-60.
- Krauss RM, Eckel RH, Howard B, et al. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000;102:2284-99.
- Aykan NF. Red Meat and Colorectal Cancer. Oncol Rev 2015;9:288.
- Karavasiloglou N, Pestoni G, Wanner M, Faeh D, Rohrmann S. Healthy lifestyle is inversely associated with mortality in cancer survivors: Results from the Third National Health and Nutrition Examination Survey (NHANES III). PloS One 2019;14:e0218048.
- Wang Y, Tao H, Paxton RJ, et al. Post-diagnosis smoking and risk of cardiovascular, cancer, and all-cause mortality in survivors of 10 adult cancers: a prospective cohort study. Am J Cancer Res 2019;9:2493-514.
- Rehm J, Shield KD, Roerecke M, Gmel G. Modelling the impact of alcohol consumption on cardiovascular disease mortality for comparative risk assessments: an overview. BMC Public Health 2016;16:363.
- Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 2015;112:580-93.
- Zarifa A, Albittar A, Kim PY, et al. Cardiac toxicities of anticancer treatments: chemotherapy, targeted therapy and immunotherapy. Curr Opin Cardiol 2019;34:441-50.
- Cohen JB, Geara AS, Hogan JJ, Townsend RR. Hypertension in Cancer Patients and Survivors: Epidemiology, Diagnosis, and Management. JACC CardioOncol 2019;1:238-51.
- Goh I, Lai O, Chew L. Prevalence and Risk of Polypharmacy Among Elderly Cancer Patients Receiving Chemotherapy in Ambulatory Oncology Setting. Curr Oncol Rep 2018;20:38.
- Wang G, Su C, Yin T. Paclitaxel and platinum-based chemotherapy results in transient dyslipidemia in cancer patients. Mol Clin Oncol 2017;6:261-5.
- Sharma M, Tuaine J, McLaren B, et al. Chemotherapy Agents Alter Plasma Lipids in Breast Cancer Patients and Show Differential Effects on Lipid Metabolism Genes in Liver Cells. PLoS One 2016;11:e0148049.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168-209.
- Oren O, Blumenthal RS, Cainzos-Achirica M, Kopecky SL. Dyslipidemia in Cancer Patients – Evolving Context, New Challenges (www.acc.org). September 4, 2019. Available at https://www.acc.org/latest-in-cardiology/articles/2019/09/04/06/43/dyslipidemia-in-cancer-patients. Accessed May 13, 2021.
- Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 2015;350:g7607.
- Johnson JA, Carstensen B, Witte D, et al. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia 2012;55:1607-18.
- Psarakis HM. Clinical Challenges in Caring for Patients With Diabetes and Cancer. Diabetes Spectr 2006;19:157-62.
- Hassan SA, Palaskas N, Kim P, et al. Chemotherapeutic Agents and the Risk of Ischemia and Arterial Thrombosis. Curr Atheroscler Rep 2018;20:10.
- Moslehi JJ. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N Eng J Med 2016;375:1457-67.
- Cuomo JR, Javaheri SP, Sharma GK, Kapoor D, Berman AE, Weintraub NL. How to prevent and manage radiation-induced coronary artery disease. Heart 2018;104:1647-53.
- Yeboa DN, Evans SB. Contemporary Breast Radiotherapy and Cardiac Toxicity. Semin Radiat Oncol 2016;26:71-8.
- Ryan AJ, Choi AD, Choi BG, Lewis JF. Breast arterial calcification association with coronary artery calcium scoring and implications for cardiovascular risk assessment in women. Clin Cardiol 2017;40:648-53.
- Quispe R, Al-Rifai M, Di Carlo PA, et al. Breast Arterial Calcium: A Game Changer in Women's Cardiovascular Health? JACC Cardiovasc Imaging 2019;12:2538-48.
- Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The Future of Concurrent Automated Coronary Artery Calcium Scoring on Screening Low-Dose Computed Tomography. Cureus 2020;12:e8574.
- Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax 2019;74:1140-6.
- Dirrichs T, Penzkofer T, Reinartz SD, Kraus T, Mahnken AH, Kuhl CK. Extracoronary Thoracic and Coronary Artery Calcifications on Chest CT for Lung Cancer Screening: Association with Established Cardiovascular Risk Factors - The "CT-Risk" Trial. Acad Radiol 2015;22:880-9.
- O'Connor SD, Graffy PM, Zea R, Pickhardt PJ. Does Nonenhanced CT-based Quantification of Abdominal Aortic Calcification Outperform the Framingham Risk Score in Predicting Cardiovascular Events in Asymptomatic Adults? Radiology 2019;290:108-15.
- Umezawa S, Higurashi T, Komiya Y, et al. Chemoprevention of colorectal cancer: Past, present, and future. Cancer Sci 2019;110:3018-26.
- Libby P, Sidlow R, Lin AE, et al. Clonal Hematopoiesis: Crossroads of Aging, Cardiovascular Disease, and Cancer: JACC Review Topic of the Week. J Am Coll Cardiol 2019;74:567-77.
- Ptashkin RN, Mandelker DL, Coombs CC, et al. Prevalence of Clonal Hematopoiesis Mutations in Tumor-Only Clinical Genomic Profiling of Solid Tumors. JAMA Oncol 2018;4:1589-93.
- Libby P, Molinaro R, Sellar RS, Ebert BL. Jak-ing Up the Plaque's Lipid Core…and Even More. Circ Res 2018;123:1180-2.
- Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017;355:842-7.
- Demissei BG, Freedman G, Feigenberg SJ, et al. Early Changes in Cardiovascular Biomarkers with Contemporary Thoracic Radiation Therapy for Breast Cancer, Lung Cancer, and Lymphoma. Int J Radiat Oncol Biol Phys 2019;103:851-60.
- Demissei BG, Hubbard RA, Zhang L, et al. Changes in Cardiovascular Biomarkers With Breast Cancer Therapy and Associations With Cardiac Dysfunction. J Am Heart Assoc 2020;9:e014708.
- Cardinale D, Stivala F, Cipolla CM. Oncologic therapies associated with cardiac toxicities: how to minimize the risks. Expert Rev Anticancer Ther 2019;19:359-74.
Clinical Topics: Cardio-Oncology, Cardiovascular Care Team, Diabetes and Cardiometabolic Disease, Dyslipidemia, Prevention, Lipid Metabolism, Nonstatins, Novel Agents, Statins, Diet, Hypertension, Smoking
Keywords: Cardiovascular Diseases, American Heart Association, Diet, Mediterranean, Sedentary Behavior, Quality-Adjusted Life Years, Risk Factors, Risk Assessment, Cardiovascular System, Myocardial Infarction, Atherosclerosis, Primary Prevention, Stroke, Neoplasms, Feeding Behavior, Biomarkers, Cholesterol, LDL, Blood Glucose, Cholesterol, HDL, Glucocorticoids, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Prediabetic State, Cardiotoxicity, Breast Neoplasms, Urinary Bladder Neoplasms, Apolipoproteins B, Paclitaxel, Blood Pressure, Body Mass Index, Diabetes Mellitus, Type 2, Hypertension, Doxorubicin, Obesity, Colorectal Neoplasms, Endometrial Neoplasms, Pancreatic Neoplasms, Decision Making, Lymphoma, Blood Glucose Self-Monitoring, Smoking
< Back to Listings