Cutting-Edge Structural Interventions | TAVR in Aortic Regurgitation: Is a "Dedicated" Device on the Horizon?
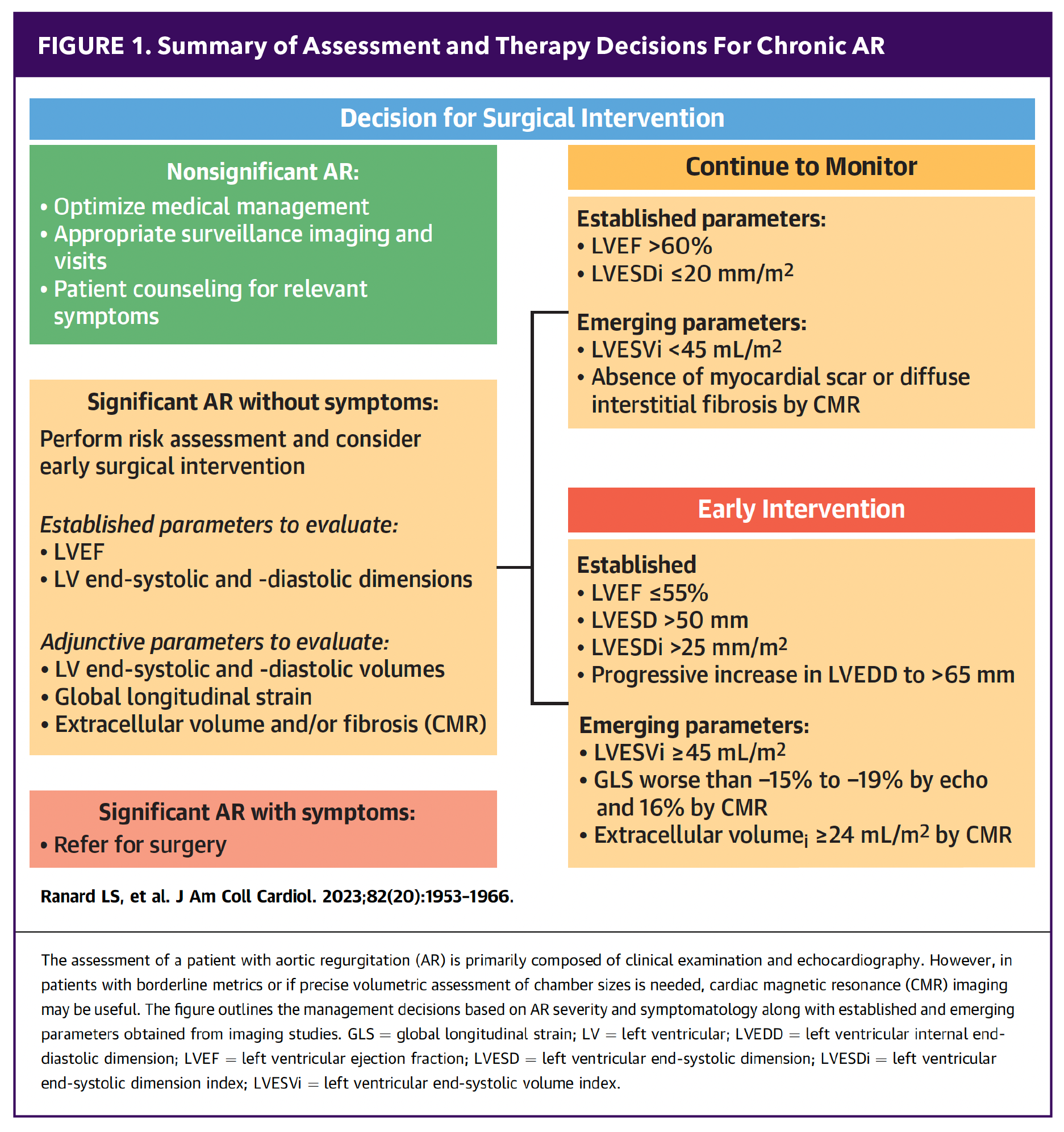
For patients with severe, pure aortic regurgitation (AR) without significant stenosis, current guidelines from both the ACC and the European Society of Cardiology (ESC) recommend surgical aortic valve replacement (SAVR) in all operable patients.1,2 Surgery is indicated for asymptomatic patients with an LVEF <55%, as well as for symptomatic patients with severe AR classified as NYHA class III-IV. It is also considered reasonable for asymptomatic patients with severe AR, an LVEF >55% and a left ventricular end-systolic diameter >50 mm.
Despite established guidelines, many patients are referred to surgery too late – often when they are no longer surgical candidates. Studies show that <25% of patients with a low LVEF receive appropriate therapy. Among patients with severe AR, only 65% undergo clinical evaluation, and a mere 3% of those with an LVEF <30% receive surgical intervention. Additionally, only 45% of patients with moderate to severe AR are referred early enough to undergo timely intervention.3 Patients with moderate AR who are undergoing other cardiac surgeries are also candidates for valve replacement, according to current recommendations (Figure 1).
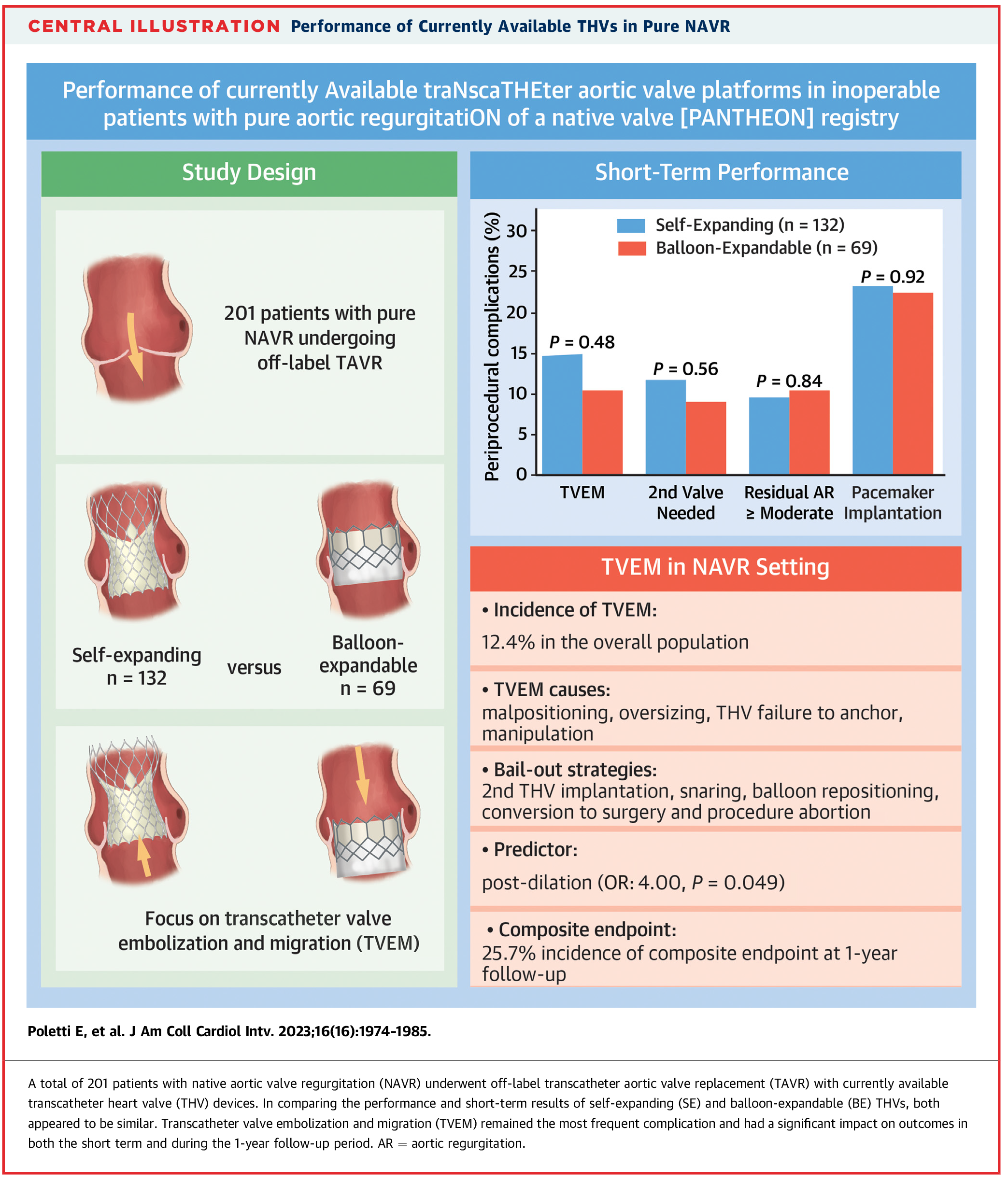
For patients who are at high or prohibitive surgical risk, TAVR has been used off-label with devices originally designed for aortic stenosis.4 However, this approach presents challenges unique to AR, including a dilated aortic root and annulus, lack of calcification for transcatheter valve anchoring and a hyperdynamic ventricle with high stroke volume – all of which can compromise valve stability and increase the risk of valve embolization or migration. In the PANTHEON study, which investigated the efficacy of self-expanding (SE) and ballon-expandable (BE) valves in high-risk AR, a technical success rate of 83.6% and a device success rate of 76.1% was reported.5 However, valve embolization occurred in 12.4% of patients, leading to rehospitalization and increased mortality (Figure 2). SE valves embolized in 13.6% of cases, compared to 10% of cases with BE valves. Notably, all embolizations occurred during the index procedure, except for one BE valve that embolized into the ventricle and was identified on six-month follow-up echocardiography. SE valves tended to embolize toward the aorta, while BE valves embolized toward the LV outflow tract. On average, the SE valves included in the study were 16% oversized compared to 8% for BE valves. It is worth noting that post-dilatation was identified as an independent predictor of valve embolization with an odds ratio of 3.4 (95% CI, 1.01-10.30, p=0.035).
In addition, a 2013 study by Roy, et al., reported 43 patients who underwent off-label CoreValve implantation for pure, severe native AR, and found residual AR in 9.2-10.1% of cases postprocedure.6
A retrospective study from Emory University compared outcomes in patients with native AR who underwent either SAVR or off-label TAVR between 2014 and 2020. Of 125 patients, 91 underwent SAVR and 34 underwent TAVR. Despite being older and having more comorbidities, TAVR patients had similar 30-day mortality rates (2.9% vs. 2.2%). At follow-up, the TAVR group showed superior hemodynamics, with larger valve areas and lower gradients. However, mitral regurgitation was more prevalent in the TAVR group (16.1% vs. 4.4%), and conduction disturbances were significantly more common, with 20.6% requiring permanent pacemaker implantation – likely due to valve oversizing for anchoring.7
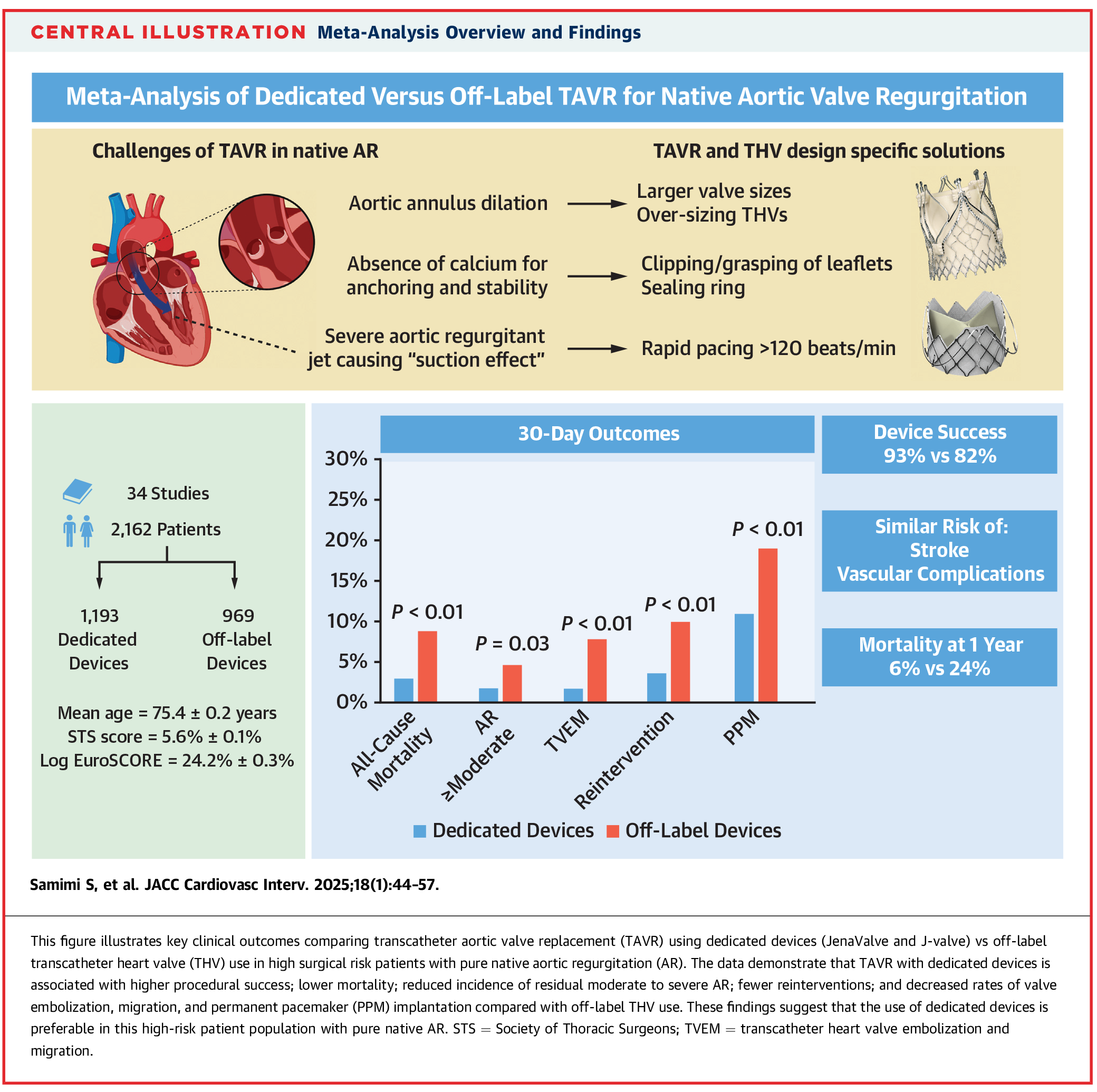
In contrast, more recent use of TAVR devices tailored for AR (JenaValve and J-Valve) have demonstrated improved outcomes. A meta-analysis conducted in 2025 reviewed 2,162 patients across 34 studies comparing off-label use with dedicated devices. High-risk patients treated with dedicated devices exhibited a lower 30-day mortality rate and a higher device success rate (93% vs. 82%; p<0.01), supporting the use of these specialized devices in this patient population8 (Figure 3).
Patient Selection For TAVR in AR
Dedicated AR TAVR devices remain under clinical investigation in the U.S. Nonetheless, selecting patients with AR for off-label AR intervention requires a careful assessment of anatomical, hemodynamic and procedural factors. While TAVR is generally reserved for patients at high surgical risk, traditional risk scores may inadequately reflect AR severity and complexity. A major factor is the underestimation of AR severity on echocardiography. Standard parameters such as pressure half-time, flow reversal and vena contracta width may not accurately grade AR severity.
Studies have shown that 20-30% of patients classified with moderate AR on transthoracic echocardiography (TTE) are reclassified as severe on cardiac MRI.3 This finding highlights the limitations of echocardiography and underscores the need for more precise diagnostic approaches. If the clinical team is unable to accurately determine AR severity, advanced imaging modalities – such as transesophageal echocardiography (TEE) or cardiac MRI – should be employed to ensure accurate assessment and prevent delays in intervention. Additionally, refining referral patterns and enhancing clinicians' understanding of the hemodynamic impact of AR will help ensure that patients receive timely and effective intervention, ultimately improving long-term outcomes.3
Trials For Dedicated Devices
JenaValve
The JenaValve Trilogy system features a clipping mechanism that anchors to the native valve leaflets, reducing migration and paravalvular leak (PVL). Data from the ALIGN-AR trial showed a 95% technical success rate and lower PVL compared to first-generation valves.9 Patients in the study were deemed high risk for open surgical valve replacement by a heart team approach.10 The device demonstrated low complication rates, with no intraprocedural deaths, a 1.4% 30-day mortality rate and minimal stroke.

Limitations include exclusion of patients with an annuli <21 mm or >28.6 mm, perimeter <66 mm or >90 mm, a dilated ascending aorta (>5.0 cm) or aortic angulation >70 degrees. Two-year follow-up data presented at ACC.25 confirmed favorable outcomes in LV remodeling, NYHA class improvement and quality of life.10 The upcoming ARTIST trial will expand eligibility to lower-risk patients, randomizing them to SAVR vs. TAVR.11
J-Valve
The J-Valve is an SE porcine valve with three U-shaped rings for anchoring and has demonstrated promising results in early feasibility studies. Eligible patients for the study must have symptomatic, severe AR and be at high surgical risk, with imaging confirming severity and anatomy suitable for valve system implantation. With a procedural success rate of 93.5% and no major complications, such as valve migration or coronary obstruction, it may prove particularly beneficial for patients with larger aortic annuli (perimeter 57-104 mm).12,13
The J-valve and ALIGN-AR studies both excluded patients with concomitant dilated ascending aorta (ascending aorta diameter >5.0 cm). Moreover, neither of these trials included patients with bicuspid aortic valves or previous prosthetic aortic valves. The latter is less of an issue due to the feasibility of using current TAVR valves inside prior failed surgical prostheses regardless of the mode of failure (recurrent stenosis vs. regurgitation).
Looking Ahead
Despite anatomical and physiological hurdles, TAVR using off-label devices has emerged as an option for high-risk patients with severe AR, comprising up to 40% of off-label TAVR procedures performed in the U.S.4 However, as clinical trials mature and device designs evolve, the use of dedicated TAVR devices for patients with AR will hopefully soon be available in the US. That will position TAVR as an alternative to surgery in carefully selected patients with AR who have an indication for treatment.

References
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632.
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary. J Am Coll Cardiol 2021;77:450-500.
- Pinto D. TCT 2024 Insights: Is Aortic Regurgitation the Next Major TAVR Target? VuMedi. November 15, 2024. Available here.
- Hira TS, Vemulapalli S, Li Z, et al.Trends and outcomes of off-label use of transcatheter aortic valve replacement: insights from the NCDR STS/ACC TVT Registry. JAMA Cardiol 2017;2:846-54.
- Poletti E, De Backer O, Scotti A. et al. Transcatheter aortic valve replacement for pure native aortic valve regurgitation: The PANTHEON International Project. J Am Coll Cardiol Intv 2023:16:1974-85.
- Roy D, Schaefer U, Guetta V, et al. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J Am Coll Cardiol 2013;61:1577-84.
- Koch R, Inci E, Grubb K, et al. A comparison of thirty-day clinical and echocardiographic outcomes of patients undergoing transcatheter vs surgical aortic valve replacement for native aortic insufficiency. Cardiovasc Revasc Med 2023; 46:85-9.
- Samimi S, Hatab T, Kharsa C, et al. Meta-analysis of dedicated vs off-label transcatheter devices for native aortic regurgitation. J Am Coll Cardiol Intv 2025;18:44-57.https://doi.org/10.1016/j.jcin.2024.08.042
- Makkar RR, Vahl TP, Thourani VH, Hamid N. Transcatheter aortic valve implantation in patients with high-risk symptomatic native aortic regurgitation (ALIGN-AR): a prospective, multicentre, single-arm study. The Lancet 2024;403:1451-9.
- Pensotti F, Valvo R, Alonge S, et al. Jena valve trilogy for the treatment of severe aortic regurgitation: a single center experience. J Am Coll Cardiol Intv 2025;18(4_Supplement) S89.https://doi.org/10.1016/j.jcin.2025.01.330.
- JenaValve Technology, Inc. JenaValve presents ALIGN-AR 2-year follow-up data at TCT 2024 and announces commencement of ARTIST randomized controlled trial (RCT). Published November 7, 2024. Accessed April 14, 2025. Available here.
- Hensey M, Murdoch DJ, Sathananthan J, et al. First-in-human experience of a new-generation transfemoral transcatheter aortic valve for the treatment of severe aortic regurgitation: the J-Valve transfemoral system. EuroIntervention 2019;14: e1557-e1559.
- Garcia S, Kaneko T, Reardon M, et al. Treatment of aortic regurgitation with a novel device: results of the j-valve early feasibility study. J Am Coll Cardiol Intv2024;17:2090-91.
Clinical Topics: Cardiac Surgery, Invasive Cardiovascular Angiography and Intervention, Valvular Heart Disease, Aortic Surgery, Cardiac Surgery and VHD, Interventions and Structural Heart Disease
Keywords: Cardiology Magazine, ACC Publications, Aortic Valve Insufficiency, Transcatheter Aortic Valve Replacement, Aortic Valve Stenosis