Cutting-Edge Structural Interventions | TAVR For Pure Native Aortic Insufficiency
Moderate or more aortic insufficiency (AI) has a prevalence of 1.6% in persons over 65 years.1 Surgical aortic valve replacement (SAVR) has long been the gold standard for treating AI. However, aortic valve replacement has been shown to be underutilized in this population. Up to 7.8% of patients with a clinical indication for SAVR receive no intervention due to older age and comorbid conditions that put them at high operative risk.2
Transcatheter aortic valve replacement (TAVR) has revolutionized the management of aortic stenosis, providing a less invasive alternative to SAVR. Its indications continue to expand to patients not studied in the original pivotal trials, including those at low surgical risk and those with end-stage renal disease, bicuspid aortic valves and failed bioprostheses.3-6 However, the road for its expansion to AI has been a rocky one.
The challenges of TAVR in pure native AI mainly stem from three unfavorable anatomical factors: 1) a lack of calcification in the aortic annulus and leaflets, 2) frequent accompanying aortic root dilation, and 3) an elliptical and large annulus.
These factors result in inadequate anchorage for available TAVR devices, leading to a high risk of valve migration, embolization and insufficient sealing. Despite these challenges, TAVR in native pure AI accounts for nearly 40% of patients undergoing off-label TAVR.7 The need for a less invasive alternative to surgery is growing, and advancements are on the horizon.
Off-Label Use of Available THV For Treating AI
Oversizing
The mainstay of off-label use of commercially available transcatheter heart valves (THV) relies on valve oversizing, allowing valve stabilization in a noncalcified annulus. Data suggest that oversizing to 15% or less predicts more than moderate residual AI, while a higher degree of oversizing (≥15%) is associated with less residual AI, particularly in self-expanding valves.8
However, such extensive oversizing carries a risk of injury to the aortic root and conduction system, as evidenced by studies consistently showing high rates of major vascular complications (~8%) and pacemaker implantation (~18%) in this population.8,9
In early experience, the self-expanding valve was preferentially used as it was believed to provide safe oversizing while minimizing the risk of aortic root rupture by relying on its radial force to seal the annulus.
However, one report has shown that the use of a newer-generation balloon-expandable valve yields at least comparable rates of device success, need for second valve implantation, residual AI, and pacemaker implantation compared with a similar-generation self-expanding valve.8
Evolving Experience
Exploratory uses of TAVR in native pure AI were reported a decade ago, but outcomes were less than optimal. The first multicenter study included 43 patients who underwent TAVR with a CoreValve (Medtronic, Minneapolis, MN) and reported high rates of residual aortic regurgitation with 18.6% of patients needing a second valve during the index procedure.10
Absence of valvular calcification was associated with the need for a second valve, highlighting that the lack of anchoring was a major drawback of TAVR in native AI. Similar results were replicated in a European cohort, which additionally reported higher mortality after TAVR for AI compared with aortic stenosis (1 month mortality: 23% vs. 3.9%; 12 months: 31% vs. 19%).11
With accumulating operator experience and improved devices, including features such as retrievability, repositioning capacity and the addition of an external skirt, TAVR in pure native AI using newer-generation THVs has shown better outcomes.8
A study including 119 patients with early-generation devices and 212 patients with newer-generation devices (note that 30.7% received a dedicated device for AI: a JenaValve and J-valve) found that TAVR in pure native AI using newer-generation vs. earlier-generation devices was associated with more devices success (81.1% vs. 61.3%), less need for a second valve implantation (12.7% vs. 24.4%) and less residual AI that was more than moderate (4.2% vs. 18.8%).8
In addition the use of newer-generation devices was associated with lower one-year cardiovascular mortality (9.6% vs. 23.6%; p=0.008). More than moderate residual AI was identified as an independent predictor of one-year all-cause mortality and rehospitalization. A systematic appraisal of 11 studies corroborated these findings.12
Contemporary National Experience
In a report from the Society of Thoracic Surgeons/ACC Transcatheter Valve Therapy Registry, AI accounted for 0.7% of all indications for TAVR performed in the U.S. in 2019.13 Among all Medicare beneficiaries who received aortic valve replacement for AI between 2016 and 2019, TAVR accounted for approximately 11% despite no recommendation from the guidelines, indicating the high demand for a less invasive strategy in this population.14
Compared with SAVR, TAVR in this study, which represents the result of off-label use of commercially available THVs, has shown lower rates of short-term complications and comparable short-term mortality but with a higher risk of mid-term mortality and reintervention even after adjustment for comorbidities.
Dedicated Transcatheter Heart Valves
JenaValve
The JenaValve (JenaValve Technology, Irvine, CA) is a self-expanding, supra-annular porcine valve characterized by its three locator clips. These clips grasp the native cusps in between the locator and sealing ring, allowing the JenaValve to anchor in a native valve without valvular calcification (Figure 1).
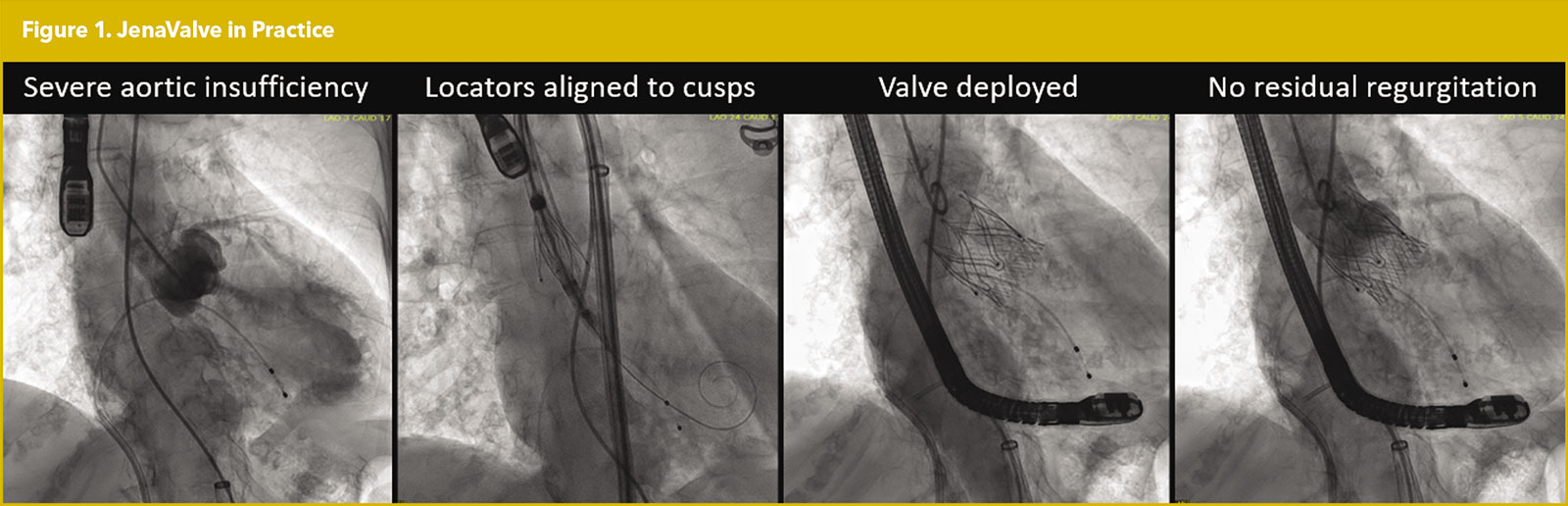
Initially a transapical approach was needed. A multicenter experience in Germany involving 31 patients demonstrated 96.8% successful implantation with no cases of more than moderate residual AI.15 However, despite good hemodynamic performance, the study reported a six-month mortality rate of 19.3%, underscoring the importance of proper patient selection and optimal timing of the procedure.
Since obtaining Conformite Europeenne (CE) mark in Europe, the JenaValve has been evaluated in the JUPITER (JenaValve EvalUation of Long Term Performance and Safety In PaTients with SEvere Aortic Stenosis or Aortic Insufficiency) registry, which prospectively enrolled 30 patients and demonstrated similar results.16
The JenaValve, compared with the first-generation CoreValve, was associated with a lower rate of more than moderate residual AI (1.6% vs. 18.2%) and need for second valve implantation (9.4% vs. 26.4%). However, stroke rates were higher (7.8% vs. 0.9%), which likely reflects the need for transapical access.8
The JenaValve can now be deployed via the transfemoral approach, and the first-in-human report has demonstrated successful deployment.17 A recent German multicenter study included 45 consecutive patients who underwent transfemoral TAVR using the JenaValve and reported 100% technical success with no deaths, strokes or conversions to open surgery.18
However the pacemaker implantation rate was high at 23%. In the U.S., the ALIGN-AR EFS (Safety and Effectiveness/Performance of the Transfemoral JenaValve Pericardial TAVR System in the Treatment of Patients With Symptomatic Severe Aortic Regurgitation) trial (NCT04415047), which is evaluating the safety and efficacy of the JenaValve in pure native AI, has completed enrollment, and its results are eagerly awaited.
J-Valve
J-Valve (JC Medical Inc, Burlingame, CA) is another self-expanding porcine valve mounted within a nitinol stent. It consists of three U-shaped anchoring rings that facilitate grasping of the native leaflets to allow anchoring in patients with pure native AI.
The transapical J-Valve has primarily been investigated in China. A study involving 134 patients with pure native AI demonstrated a device success rate of 96.3%, with only 0.7% experiencing more than moderate residual AI and six-month mortality of 3.7%.19 A multicenter study involving 44 patients reported a similar success rate, with a two-year mortality rate of 11.6%.20 A transfemoral device is under development.19 However, the J-Valve is currently not available in the U.S.
Looking Ahead
TAVR in AI remains challenging, especially with currently available THVs. Dedicated devices such as the JenaValve and J-Valve have shown encouraging results and will almost certainly play a large role in the treatment of patients with pure AI in the future. A safe and effective transcatheter strategy seems just around the corner in the U.S.


This article was authored by Hiroki Ueyama, MD, and Isida Byku, MD, FACC, from the Division of Cardiology, Emory Structural Heart and Valve Center, Emory University Hospital Midtown, in Atlanta, GA.
References
- d'Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: The OxVALVE population cohort study. Eur Heart J 2016;37:3515-22.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart survey on valvular heart disease. Eur Heart J 2003;24:1231-43.
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695-1705.
- Mentias A, Desai MY, Saad M, et al. Management of aortic stenosis in patients with end-stage renal disease on hemodialysis. Circulation: Cardiovasc Interv 2020;13:e009252.
- Vincent F, Ternacle J, Denimal T, et al. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Circulation 2021;143:1043-61.
- Tuzcu EM, Kapadia SR, Vemulapalli S, et al. Transcatheter aortic valve replacement of failed surgically implanted bioprostheses: The STS/ACC Registry. J Am Coll Cardiol 2018;72:370-82.
- Hira RS, Vemulapalli S, Li Z, et al. Trends and outcomes of off-label use of transcatheter aortic valve replacement: Insights from the NCDR STS/ACC TVT Registry. JAMA Cardiol 2017;2:846-54.
- Yoon S-H, Schmidt T, Bleiziffer S, et al. Transcatheter aortic valve replacement in pure native aortic valve regurgitation. J Am Coll Cardiol 2017;70:2752-63.
- Sawaya FJ, Deutsch M-A, Seiffert M, et al. Safety and efficacy of transcatheter aortic valve replacement in the treatment of pure aortic regurgitation in native valves and failing surgical bioprostheses: Results From an international registry study. JACC: Cardiovasc Interv 2017;10:1048-56.
- Roy DA, Schaefer U, Guetta V, et al. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J Am Coll Cardiol 2013;61:1577-84.
- Testa L, Latib A, Rossi ML, et al. CoreValve implantation for severe aortic regurgitation: a multicentre registry. EuroIntervention 2014;10:739-45.
- Takagi H, Hari Y, Kawai N, Ando T. Meta-analysis and meta-regression of transcatheter aortic valve implantation for pure native aortic regurgitation. Heart Lung Circ 2020;29:729-41.
- Carroll JD, Mack MJ, Vemulapalli S, et al. STS-ACC TVT Registry of transcatheter aortic valve replacement. J Am Coll Cardiol 2020;76:2492-2516.
- Mentias A, Saad M, Menon V, et al. Transcatheter vs surgical aortic valve replacement in pure native aortic regurgitation. Ann Thorac Surg 2023;115:870-76.
- Seiffert M, Bader R, Kappert U, et al. Initial German experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv 2014;7:1168-74.
- Silaschi M, Conradi L, Wendler O, et al. The JUPITER registry: One-year outcomes of transapical aortic valve implantation using a second generation transcatheter heart valve for aortic regurgitation. Catheter Cardiovasc Interv 2018;91:1345-51.
- Schäfer U, Schirmer J, Schofer N, et al. First-in-human implantation of a novel transfemoral self-expanding transcatheter heart valve to treat pure aortic regurgitation. EuroIntervention 2017;13:1297-1300.
- Tammer A. JenaValve trilogy for treatment of aortic regurgitation: worldwide first results. Presented at: EuroPCR 2022. May 18, 2022. Paris, France.
- Liu L, Chen S, Shi J, Qin C, Guo Y. Transcatheter aortic valve replacement in aortic regurgitation. Ann Thorac Surg 2020;110:1959-65.
- Shi J, Wei L, Chen Y, et al. Transcatheter aortic valve implantation with J-Valve: 2-Year outcomes from a multicenter study. Ann Thorac Surg 2021;111:1530-6.
Clinical Topics: Cardiac Surgery, Congenital Heart Disease and Pediatric Cardiology, Invasive Cardiovascular Angiography and Intervention, Valvular Heart Disease, Aortic Surgery, Cardiac Surgery and CHD and Pediatrics, Cardiac Surgery and VHD, Congenital Heart Disease, CHD and Pediatrics and Interventions, Interventions and Structural Heart Disease
Keywords: ACC Publications, Cardiology Magazine, Innovation, Transcatheter Aortic Valve Replacement, Heart Valve Prosthesis, Bioprosthesis, Patient Readmission, Dilatation, Prevalence, Aortic Valve Stenosis, Aortic Valve Insufficiency, Bicuspid Aortic Valve Disease, Artificial Intelligence
< Back to Listings


