Cover Story | Transcatheter Mitral Valve Therapies: The Next Frontier
It seems that almost overnight, transcatheter aortic valve replacement has become yesterday’s news and the interventional cardiology world has turned its attention upstream to the mitral valve and its regurgitant tendencies.
Transcatheter mitral valve repair (TMVr) and replacement (TMVR) are transforming the treatment of mitral regurgitation (MR). They also have the biotech community buzzing with excitement and dollars.
The ACC has responded to this surge in interest by releasing an Expert Consensus Decision Pathway to guide clinicians caring for patients with MR (see sidebar). This new document, plus a slew of the newest trial findings, will be the topic of this review.
 Image courtesy of Abbott Structural Heart
Image courtesy of Abbott Structural Heart
MR: The Forgotten Disease
The unmet need is indisputable: mitral regurgitation is the most common type of moderate or severe heart valve disease among U.S. adults older than 55 years and its prevalence is increasing. The hemodynamic effects of MR can be severe due to the pure volume overload it imposes on the left ventricle (LV). Debilitating heart failure and atrial fibrillation (AFib) are commonly seen comorbidly with MR.
“The mitral valve is a complex structure and mitral valve disease is a heterogeneous entity,” explained Bernard Prendergast, MD, during a TCT 2017 session devoted to the topic of transcatheter mitral interventions.
MR is broadly categorized as either primary (or degenerative) or secondary (or functional) MR. Primary MR is caused by an anatomical abnormality of the valve that prevents the leaflets from coapting properly. This might be leaflet thickening, calcification, perforation or prolapse, or it might refer to issues of the subvalvular apparatus, including rupture, detachment, thickening or fusion of the chordae tendinae.
Secondary MR occurs when the leaflets are normal but fail to coapt properly because of changes to the left ventricular and mitral annulus brought by ischemic or nonischemic dilated cardiomyopathies. Fully 90 percent of all MR is functional.
Clinically, MR is often overlooked and undertreated. Mitral valve surgery (either repair or replace) is the gold standard of care for patients with degenerative MR. But the vast majority do not undergo surgery for reasons that are not completely understood. In the case of secondary MR, a mortality benefit from surgery has not been demonstrated and only a small proportion of patients with isolated functional MR are operated on.
Just as MR can be caused by more than one valve abnormality, TMVr devices target different aspects of mitral valve pathology: edge-to-edge leaflet repair, direct and indirect annuloplasty, chordal repair and valve spacers.
MitraClip Rules in Edge-to-Edge Repair
With a U.S. Food and Drug approval and more than 45,000 treated patients worldwide, MitraClip is the transcatheter mitral intervention that all the other interventions want to be. The most recent data on MitraClip comes from the Society of Thoracic Surgery (STS)/ACC Transcatheter Valve Therapy registry.1 The study population consisted of 2,952 patients treated at 145 U.S. hospitals between November 2013 and September 2015.
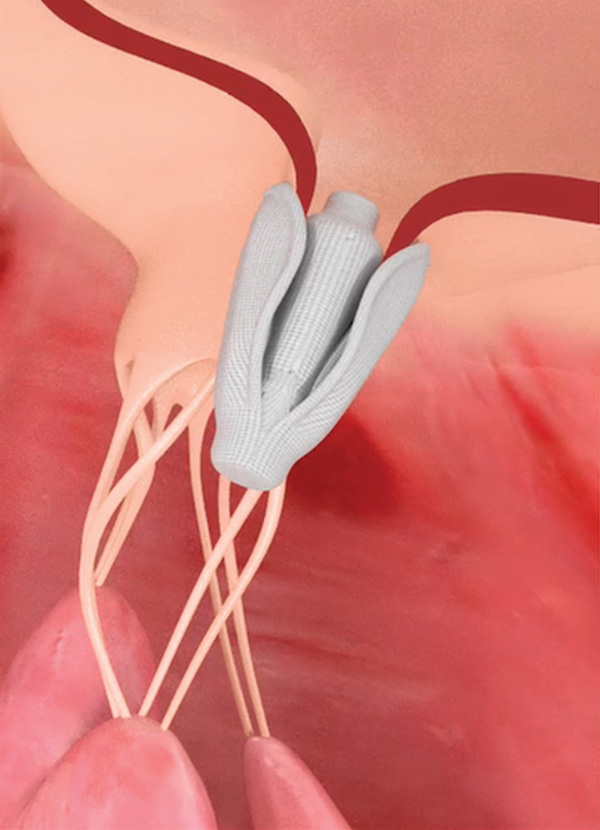 Image courtesy of Edwards Lifesciences, Inc.
Image courtesy of Edwards Lifesciences, Inc.
Most patients (85.9 percent) had degenerative MR. Functional MR was described in 17.5 percent, including 262 patients in whom degenerative disease was concurrently reported (8.9 percent of total patients). Just over one-third of patients received two or more clips.
In data reflective of real-world MitraClip use, Paul Sorajja, MD, FACC, and colleagues reported in-hospital mortality of 2.7 percent and acute procedural success of 91.8 percent. Post-procedural MR of 2+ or more was seen in 40 percent of MitraClip recipients.
Among patients with outcomes data, mortality at 30 days and one year were 5.2 percent and 25.8 percent, respectively, and repeat hospitalization for heart failure at one year was seen in 20.2 percent.
“Although TMVR is associated with a high rate of procedural success in the United States, roughly one in five patients are rehospitalized with heart failure, and one in four do not survive beyond one year,” wrote Sorajja and colleagues.
Patients with worse post-procedural MR, functional MR and untreated tricuspid regurgitation had worse outcomes.
“When you look at the outcomes at a year, they’re horrible,” said Martin B. Leon, MD, FACC, co-director of the TCT meeting, noting, for example, the 49.0 percent cumulative incidence of death or heart failure hospitalization in the functional MR group.
The device is only approved for primary MR patients who are not operative candidates, but it’s currently being tested in the COAPT trial against guideline-directed medical therapy in patients with functional MR. Results are expected in fall 2018 and should offer insight into whether MitraClip improves survival in these patients.
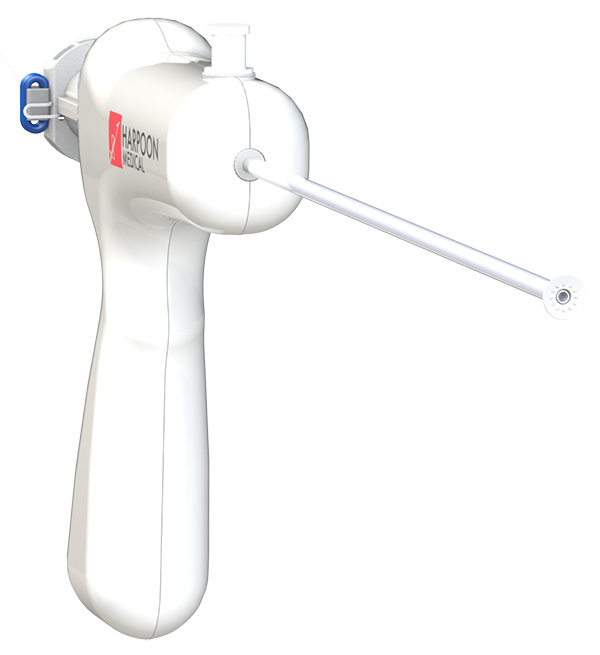 Image courtesy of Harpoon Medical, Inc.
Image courtesy of Harpoon Medical, Inc.
“In Europe, there are a lot of patients already being treated with MitraClip for functional MR,” said Christian Shults, MD, during an AHA 2017 Annual Scientific Sessions presentation. “I think that will be the trend for it along with other therapies that potentially offer an effective solution for patients with functional MR.”
A device that works similarly to the MitraClip is the Edwards PASCAL mitral valve repair system. The device is designed to extend interventional treatment of patients with MR who are not well served by MitraClip. As such, it simplifies navigation in the left atrium, allows for independent leaflet grasping and improves MR reduction through implementation of a central spacer.
In a recent multicenter trial in 23 patients who received the device for compassionate use due to ineligibility for MitraClip, successful implantation of at least one device was accomplished in all patients, resulting in procedural residual MR grade 2+ or less in 96 percent of patients.2
Three patients (13 percent) died during the 30-day follow-up, all of cardiovascular causes. Of those alive at 30 days, 80 percent were in NYHA class II heart failure and 15 percent had NYHA class I heart failure. Grade 1+ MR was found in 63 percent of patients. The ongoing CLASP study will see the PASCAL device used in 130 patients and include three-year follow-up.
Fixing The Ties That Bind
Another target for TMVr are the fibrous chordae tendinae, which with their delicate microstructure are responsible for determining the position and tension on the anterior and posterior leaflets at LV end-systole.
The TRACER trial – just presented at TCT 2017 – tested the Harpoon (Harpoon Medical, Inc., Baltimore, MD) mitral valve repair system in 30 patients with severe, degenerative MR. Harpoon is a transventricular, echo-guided device designed to implant artificial expanded polytetrafluoroethylene (ePTFE) cords on mitral leaflets in the beating heart. Although not technically a transcatheter device, it offers a simplified off-pump repair of degenerative MR with real-time titration of ePTFE cordal length to maximize coaptation.
The device’s inventor, James S. Gammie, MD, FACC, is a cardiac surgeon from the University of Maryland. The Harpoon is designed to be used by surgeons, not interventionalists. In TRACER, technical success was seen in 93 percent (28 of 30). Two patients required intraoperative conversion to conventional surgery. MR was reduced to none or trace in 86 percent of patients and mild in 14 percent. Two patients developed new postoperative AFib, but there were no deaths, strokes, pacemaker implantations or incident renal failure.
At six months, MR was ≤ mild in 85 percent, moderate in eight percent and severe in eight percent. All patients were in NYHA class I heart failure at six months. Favorable remodeling included decreases in end-diastolic and anterior-posterior mitral dimensions. At six-month follow-up, mean LV ejection fraction was 66 percent.
“We anticipate CE Mark approval in the very near future,” reported Gammie. Harpoon has received financial support from Edwards Lifesciences, which also holds an option to acquire Harpoon.
Cinching Up the Annulus
 Image courtesy of Edwards Lifesciences, Inc.
Image courtesy of Edwards Lifesciences, Inc.
Since it was initially invented 40 years ago by Alain Carpentier, MD, PhD, FACC, mitral valve annuloplasty has been a standard tool for surgeons. A number of complimentary transcatheter devices are under investigation, including the Cardioband (Valtech Cardio – Edwards Lifesciences), a transcatheter surgical-like direct mitral valve annuloplasty system.
“Cardioband is a technology delivered from the femoral vein, transeptal puncture, and it cinches the annulus under transesophageal echo guidance to reduce the mitral regurgitation,” explained Shults. “What’s interesting is the idea of combo therapy – belt and suspenders – using the NeoChord, Harpoon or MitraClip to fix the degenerative problem, but also adding Cardioband or some other kind of annuloplasty device to treat the overall mitral valve,” he said.
At TCT 2017, Stephen G. Worthley, MBBS, PhD, FACC, presented six-month outcomes from the MAVERIC trial of the ARTO system in 45 patients with functional MR. A 100 percent device success was achieved, with only two adverse events during the initial 30 days (one tamponade, one renal failure). The ARTO is a transcatheter annular reduction therapy system developed by MVRx, Inc. It is designed to address annular dilatation in symptomatic functional MR patients by pulling the lateral wall to the septum, thereby reducing the mitral valve anterior-posterior dimension.
MAVERIC reported 100 percent device success and no death or stroke at 30 days. By six months, the rate of the composite safety endpoint was 16.0 percent (seven events), which included three cardiac deaths. No deaths were attributed to the device of the procedure at six months.
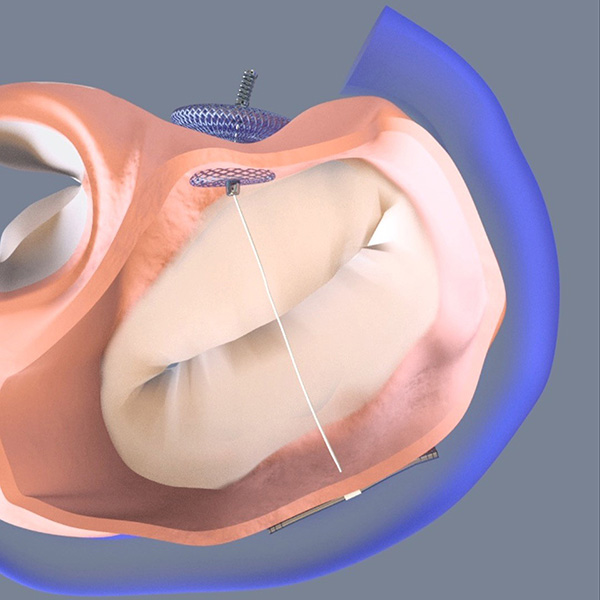 Image courtesy of MVRx, Inc.
Image courtesy of MVRx, Inc.
Fully 90 percent of patients had MR 2+ or less and the MR reduction seen at 30 days was maintained at six months. “In fact, sixty percent of patients had only trace or only mild MR,” reported Worthley. He stressed the improvements seen in functional status: 78 percent of patients were in NYHA class I or II heart failure at six months, a dramatic improvement from the 71 percent in NYHA class III or IV heart failure at baseline.
“Going forward, there will be some refinements to the procedure to make it easier and shorter… and the next step will be a larger scale trial looking at not just these surrogate outcomes such as ventricular function and symptoms, but harder endpoints such as rehospitalization and mortality,” Worthley told the ACC’s On The Scene.
TMV-Replacement Rises
After some early setbacks, TMVR is advancing steadily, albeit with small individual studies and an overall studied population that still numbers well under 500 patients worldwide. Two trials – Tendyne and Intrepid – reported findings at TCT 2017, highlighting just two of several devices in development for this indication.
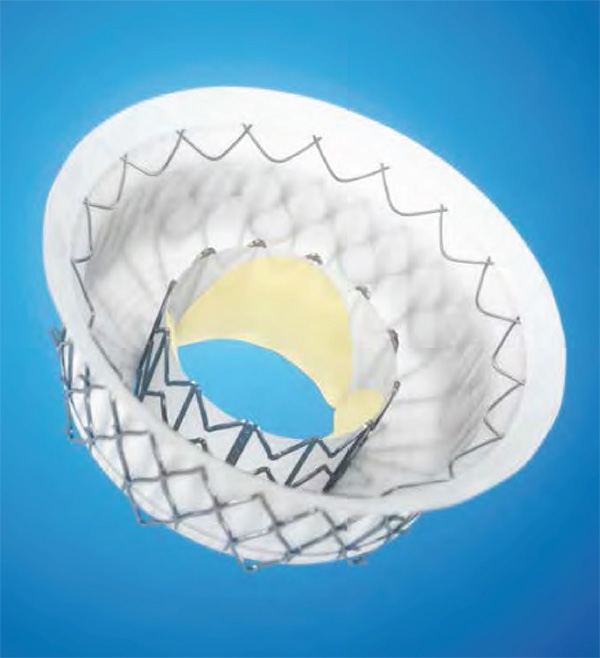
Sorajja presented early findings for the Intrepid TMVR system (Medtronic), which were also published online in the Journal of the American College of Cardiology.3 The valve is a trans-apically delivered, self-expanding, nitinol device with bovine pericardial leaflets.
This global pilot study enrolled 50 patients, 84 percent of whom had secondary MR. Mean LV ejection fraction was 43 percent and 86 percent of patients had NYHA class III or IV symptoms. Implant success was seen in all but two patients (96 percent).
At 30-days, mortality was 14 percent. Three of the seven deaths were related to apical access site bleeding at or immediately after the initial procedure, one was in a patient with malposition and three other patients died of refractory heart failure early after the procedure. There were no disabling strokes or repeat interventions.
At the latest follow-up (median 173 days), echocardiography confirmed mild or no residual MR in all implanted patients. Significant improvements in symptom class (79 percent in NYHA I or II at follow-up; p < 0.0001 vs. baseline) and Minnesota heart failure questionnaire scores (p = 0.011) were observed.
“As noted by the INTREPID investigators, these findings are provocative,” said ACC.org Editor-in-Chief Kim A. Eagle, MD, MACC. “However, it’s important to remember they are also preliminary. Outcomes from larger, longitudinal studies will be important to watch in the coming years.”
The Tendyne mitral valve (Abbott Structural Heart) is a tri-leaflet porcine pericardial valve that is also delivered via trans-apical access. The valve features adjustable tension to provide stability and has a self-expanding nitinol frame. In the Tendyne feasibility study, the valve was implanted in 30 patients; three with primary MR, 24 with secondary MR and three with mixed pathology. The vast majority (93.1 percent) had grade 4+ MR at baseline. Implant success was achieved in 28 patients.
At one year, mortality was seen in 16.7 percent of patients, 13.3 percent of which was cardiac in nature. There was one case of valve malposition/paravalvular leak/hemolysis and one case of leaflet thrombosis. Overall, 21 of 22 patients with one-year follow-up had no MR symptoms and 19 of 20 patients improved to NYHA functional class I or II.
 Image courtesy of Abbott Structural Heart
Image courtesy of Abbott Structural Heart
“It works,” said Peter C. Block, MD, FACC, in an ACC On The Scene video. “There’s no question that Tendyne is probably a pretty good valve and in some patients seems to make a lot of difference in the reduction of mitral regurgitation. But I worry about Tendyne, because patients with MR usually have dilated left ventricles and if you put a hole in the left ventricular apex, I can’t imagine that’s good for the left ventricle.”
In a TCT Talk, Leon stressed that the technology needs to advance from trans-apical approaches to trans-septal approaches. “Trans-septal, which is transfemoral, TMVR has clear safety advantages. Currently, there are technical limitations in device size, system flexibility, steerability, and alignment, but these will be overcome in the foreseeable future.”
Repair or Replace, Percutaneously Speaking?
One question unanswered in the mitral arena is whether transcatheter repair will be better, worse or equal to transcatheter replacement. Surgically speaking, repair is recommended when possible, but will the same hold true for percutaneous mitral valve interventions?
In a TCT 2017 flash debate on mitral valve repair or replacement, Leon had a clear message for attendees: “The issue is that there is no good transcatheter repair, and if there is no good repair, then replacement is better than a bad repair,” [emphasis his].
“The promise of TMVR is that we can make it less invasive than open surgery. Hopefully much safer, reduce complications, shorter recovery times, make it predictable with durable elimination of all MR and no recurrence, and then more generalizable to transcatheter operators if we can get over the technical challenges and make this a transfemoral procedure,” Leon said.
And if the promise is fulfilled, then, said Leon, “we can treat all these patient subsets: high surgical risk for thoracotomy, alternative to surgical MVR, recurrence after surgical repair, unable to perform surgical repairs, some [mitral annular calcification] patients, and some rheumatic heart disease patients.” Also, TMVR has the potential to offer an all-in-one fix that addresses all the heterogeneous pathologies of the mitral valve, said Leon.
While it’s still early days, hope abounds…with the oft-repeated caveat that Prendergast put so succinctly: “I think we need to recognize that the runaway success story of TAVI is unlikely to be replicated anytime soon.”
References
- Sorajja P, Vemulapalli S, Feldman T, et al. J Am Coll Cardiol 2017;70:2315-27.
- Praz F, Spargias K, Chrissoheris M, et al. Lancet 2017;390:773-80.
- Bapat V, Rajagopal V, Meduri C, et al. J Am Coll Cardiol 2017;Oct 25:[Epub ahead of print].
Clinical Topics: Arrhythmias and Clinical EP, Cardiac Surgery, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Valvular Heart Disease, Atrial Fibrillation/Supraventricular Arrhythmias, Cardiac Surgery and Arrhythmias, Cardiac Surgery and Heart Failure, Cardiac Surgery and VHD, Acute Heart Failure, Interventions and Imaging, Interventions and Structural Heart Disease, Echocardiography/Ultrasound, Mitral Regurgitation
Keywords: ACC Publications, Cardiology Interventions, Alloys, Atrial Fibrillation, Biotechnology, Cardiac Surgical Procedures, Cardiomyopathy, Dilated, Chordae Tendineae, Compassionate Use Trials, Dilatation, Drug Approval, Echocardiography, Feasibility Studies, Femoral Vein, Financial Support, Heart Atria, Heart Failure, Heart Valve Diseases, Heart Ventricles, Hemodynamics, Hemolysis, Hospital Mortality, Hospitalization, Incidence, Longitudinal Studies, Mitral Valve, Mitral Valve Annuloplasty, Mitral Valve Insufficiency, Pacemaker, Artificial, Pilot Projects, Polytetrafluoroethylene, Prevalence, Prolapse, Punctures, Registries, Renal Insufficiency, Research Personnel, Rheumatic Heart Disease, Standard of Care, Stroke, Stroke Volume, Surgeons, Surgical Instruments, Systole, Thoracic Surgery, Thoracotomy, Thrombosis, Transcatheter Aortic Valve Replacement, Tricuspid Valve Insufficiency, Ventricular Function
< Back to Listings


