Current State of MRI With Cardiac Devices
Quick Takes
- Magnetic resonance imaging (MRI) examinations in patients with cardiac implantable electronic devices (CIEDs) are safe if safety protocols are followed with predevice and postdevice checks, and when there is adequate supervision and monitoring.
- In MRI-conditional CIEDs, scanning protocols should follow manufacturer recommendations.
- Novel technical advances have increased the diagnostic yield of MRI by reducing CIED-related artifacts.
Magnetic resonance imaging (MRI) is increasingly performed each year due to expanding indications, both cardiac and noncardiac, and to enhanced access. The same drivers have increased the use of cardiac implantable electronic devices (CIEDs). It is estimated that one in two patients with CIEDs will require an MRI following device implantation. The magnetic field generated by an MRI scanner has generated safety concerns because of potential interactions with components of the CIED leading to mechanical force and torque, overheating of CIED components, current induction leading to myocardial capture at fast rates, power-on reset with inappropriate inhibition of pacing and/or delivery of tachy-therapies, and CIED malfunction or loss of function.
Therefore, "MRI-conditional" devices were purposely designed to circumvent potential harm during an MRI study. The results of large studies from the authors' group and others have also demonstrated overall safety of MRI examinations using 1.5 Tesla (1.5T) scanners in patients with non–MRI-conditional devices (termed "legacy" devices) provided prespecified safety protocols are followed. These studies were performed following the publication of in vitro and in vivo data showing that, given clinical conditions, lead-tip heating could be limited to <0.5°C and magnetic field-induced current to <0.5 mA. Meanwhile, cardiac MRI sequences such as wideband inversion pulse sequences have been developed to reduce image artifacts and increase diagnostic utility in patients with CIEDs.
As such, many cardiovascular (CV) societies have supported the use of MRI in patients with MRI-conditional and non–MRI-conditional devices and have released guidelines outlining proper safety protocols to prevent negative outcomes. Despite vast advancements in both MRI and CIED technologies and overwhelming evidence of MRI safety for CIEDs, these patients still experience delayed access to or lack proper access to MRIs because of the lack of personnel, lack of technical resources, or knowledge gaps in the latest evidence. Two major CV societies, the Society of Cardiac Magnetic Resonance (SCMR) and the Joint British Society (JBS), have recently released updated guidelines on the topic, which will be highlighted in this discussion.1,2
MRI-conditional labeling is provided by the CIED manufacturers and relates to design features intended to reduce interaction with magnetic fields. These features include coil configuration, heat-dissipating filters, nonferromagnetic materials, and programming features. Even if individual components of the system are MRI-conditional, mismatched components from different vendors render the system non-MRI conditional despite a lack of evidence of increased risk. According to the 2024 SCMR expert consensus document, there are three categories of patients with CIEDs with increasing MRI-related levels of risk, including those with1:
- MRI-conditional CIED systems
- Non–MRI-conditional systems without abandoned, fractured, or epicardial leads (including MRI-conditional CIEDs with non–MRI-conditional leads or generators)
- Patients with any CIED with abandoned or fractured intracardiac leads or epicardial permanent leads.
However, it is important to note that, despite theoretical risks, patients in all these categories can undergo safe MRI given appropriate precautions.3,4
Performing an MRI examination on patients with any CIED requires: a team-based, collaborative enterprise between cardiology/electrophysiology and radiology; appropriate monitoring equipment; and the ability to perform prescan and postscan device assessments (Figure 1). Appropriate review of the device should be performed prior to the scan to establish MRI-conditional status and to assess for epicardial, abandoned, or fractured leads on chest imaging. It is paramount to review indications for the scan, the anticipated diagnostic yield based on CIED location, alternative imaging modalities, pacing dependence status, generator battery life, and recent arrhythmias that may impact the safety of temporarily withholding therapies during MRI. Using 1.5T scanners is recommended for non–MRI-conditional CIEDs. Manufacturer recommendations on specific absorption rate, body position, and scanner strength should be followed for MRI-conditional devices.
Figure 1: Approach to Reviewing MRI-Conditional and non–MRI-Conditional CIEDs Before, During, and After MRI Studies
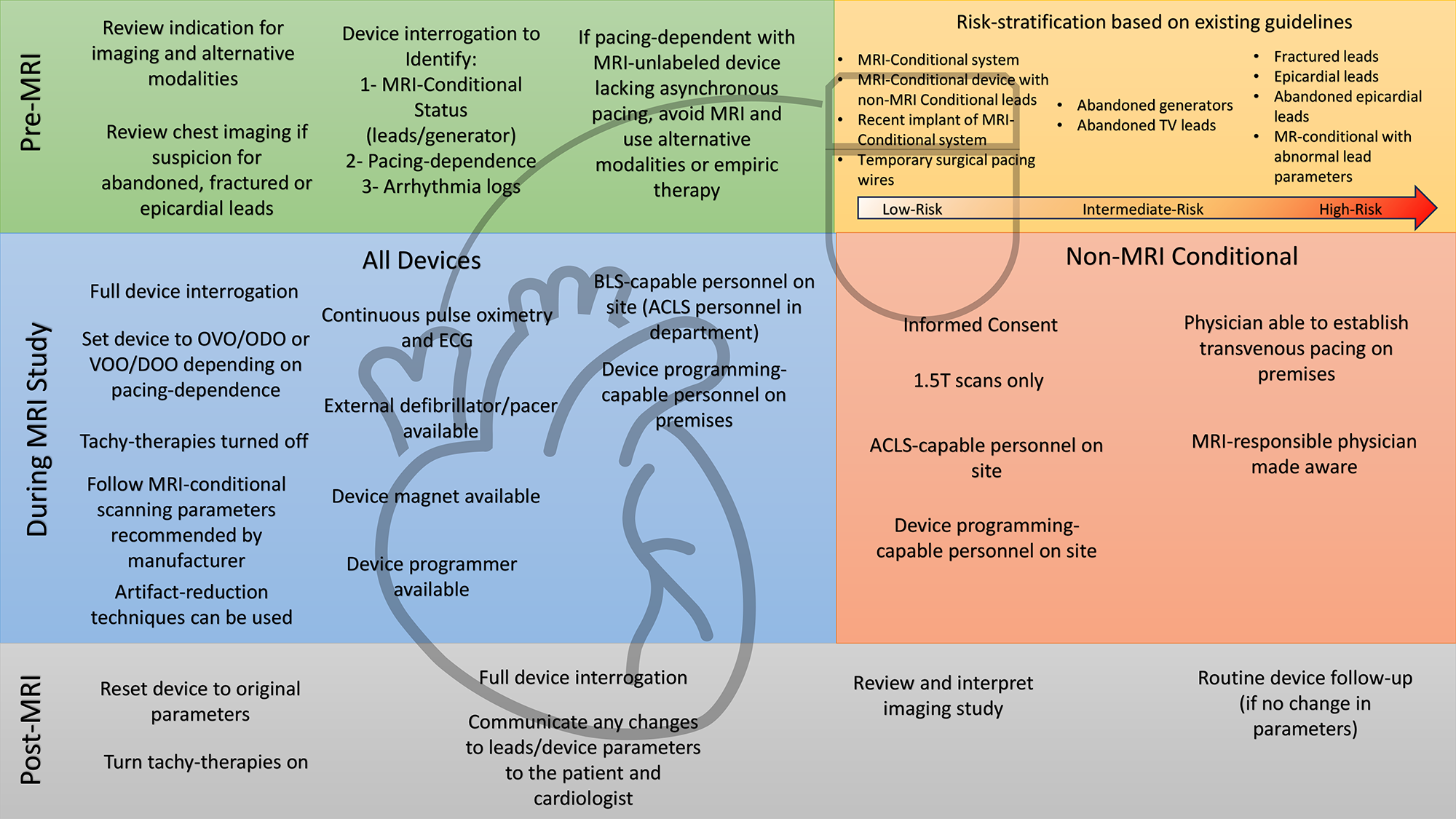
1.5T = 1.5 Tesla; ACLS = advanced cardiac life support; BLS = basic life support; CIEDs = cardiac implantable electronic devices; ECG = electrocardiogram; MR = magnetic resonance; MRI = magnetic resonance imaging; TV = transvenous.
The recommended safety protocols are similar in both guideline documents. In summary, on the day of the scan, informed consent must be obtained from patients with non–MRI-conditional CIEDs. MRI-conditional pulse oximetry, electrocardiogram telemetry, and blood pressure monitors should be applied for the duration of the scan. A device programmer, resuscitation cart (including an external defibrillator with transcutaneous pacing capabilities), and advanced cardiac life support–certified personnel should be available on premises. Devices should be programmed to MRI-safe mode, which consists of "OVO" or "ODO" in patients who are not pacing dependent and asynchronous "VOO" or "DOO" mode in those who are pacing dependent. Tachy-therapies are temporarily disabled in implantable cardiac defibrillators to prevent inappropriate therapies and battery depletion. After scanning is finished, a complete device interrogation is performed with restoration of the original parameters. Lead sensing, pacing thresholds, and impedance are measured, and any significant change is discussed with a cardiologist. For emergency MRI referrals, safety protocols should not be bypassed because performing a scan without appropriate monitoring and device management is contraindicated. If appropriate resources are unavailable, alternative imaging modalities should be ordered or patients should be referred to another facility.
Abandoned/fractured/epicardial leads have historically been deemed particularly high risk, leading to exclusion from the 2017 Heart Rhythm Society (HRS) consensus document and from Centers for Medicare and Medicaid Services (CMS) reimbursement eligibility. However, the results of several recent studies have demonstrated no serious safety events (device malfunction, myocardial injury, arrhythmias, etc.) associated with MRI examinations in patients with abandoned leads, including epicardial leads.3 Abandoned leads have been downgraded to intermediate risk in recent guidelines whereas fractured leads are still considered high risk. In these cases, individualized decisions should be made after weighing the risk/benefit ratio of the MRI and considering alternative imaging modalities to answer the clinical question. The SCMR recommends referring these patients to experienced centers only.
In conclusion, MRI examinations in patients with CIEDs are safe when rigorous protocols are followed regardless of the type of CIED. Categories previously considered high risk, such as abandoned or epicardial leads, have shown low rates of adverse outcomes. The risks of pursuing invasive tests, delaying treatments because of missed diagnoses, or extracting non–MRI-conditional leads should push referring clinicians, hospital administrators, and health care payors to support the establishment of accessible MRI programs for patients with CIEDs.
References
- Kim D, Collins JD, White JA, et al. SCMR expert consensus statement for cardiovascular magnetic resonance of patients with a cardiac implantable electronic device. J Cardiovasc Magn Reson 2024;Jan 12:[ePub ahead of print].
- Bhuva A, Charles-Edwards G, Ashmore J, et al. Joint British Society consensus recommendations for magnetic resonance imaging for patients with cardiac implantable electronic devices. Heart 2024;110:[ePub ahead of print].
- Schaller RD, Brunker T, Riley MP, Marchlinski FE, Nazarian S, Litt H. Magnetic resonance imaging in patients with cardiac implantable electronic devices with abandoned leads. JAMA Cardiol 2021;6:549-56.
- Ra J, Oberdier MT, Suzuki M, et al. Implantable defibrillator system shock function, mortality, and cause of death after magnetic resonance imaging. Ann Intern Med 2023;176:289-97.
Clinical Topics: Arrhythmias and Clinical EP, Noninvasive Imaging, Implantable Devices, SCD/Ventricular Arrhythmias, Magnetic Resonance Imaging
Keywords: Magnetic Resonance Imaging, Implantable Devices, Defibrillators, Implantable, Cardiac Resynchronization Therapy Devices, Pacemaker, Artificial
