Multimodality Imaging in Pericardial Diseases and the Role of Imaging-Guided Therapies
Quick Takes
- Multimodality imaging is critical for the diagnosis, prognostication, and monitoring of treatment response in the setting of different pericardial pathologies and offers the ability to predict future risk of recurrences.
- Improved understanding of the pathogenesis of pericardial injury/inflammation, inflammasome polymerization, and interleukin-1 (IL-1) secretion have paved the way to specialized treatment strategies.
- Imaging-guided therapies allow for prompt and correct application of personalized treatment plans using nonsteroidal anti-inflammatory drugs/aspirin, corticosteroids, colchicine, and the novel IL-1 blockers according to each patient phenotype and their respective imaging findings.
Introduction
Pericardial diseases represent the second-most common cause of emergency department and acute care visits for chest pain. Despite their significant prevalence, there are substantial educational gaps and inconsistent approaches to diagnosis and management of these conditions. Until recently,1 there have been no updated guidelines addressing these conditions, and the most recent European guidelines published in 2015 do not reflect contemporary clinical practice. From this perspective, the recent international position statement on new concepts and advances in multimodality cardiac imaging in pericardial diseases aims to inform clinicians about new concepts and advances in cardiac multimodality imaging (cMMI) and their applications in the diagnosis, risk stratification, surveillance, and management of complex pericardial diseases.1 This article provides an expert review of this document, with a special focus on the role of integrated cMMI and the use of imaging-guided therapies (IGT).
Acute Pericarditis
Acute pericarditis (AP) is a clinical syndrome driven by various pathogenetic mechanisms, with interleukin-1 (IL-1) playing a central role in the inflammatory phenotype.2 Transthoracic echocardiography (TTE) is the first-line imaging modality and is mainly used to identify the presence and size of a pericardial effusion (PEff), obtain findings of constrictive pericarditis (CP), and evaluate myocardial involvement in AP.1 Transesophageal echocardiography (TEE) is typically reserved for cases in which TTE provides suboptimal acoustic windows or is not feasible, such as during or immediately following cardiac surgery. TEE offers excellent spatial resolution, high reliability, and accuracy in detecting pericardial thickening compared with TTE.1 Cardiac computed tomography, a second-line imaging modality, has a limited role in AP but is occasionally useful for detecting pericardial thickening and excluding associated and/or alternative thoracic pathologies.3 Cardiac magnetic resonance (CMR) is another second-level imaging modality that is indicated in high-risk AP, if myocardial involvement is suspected, and in the setting of AP with a noninflammatory phenotype. The value of CMR stems from its tissue characterization via black-blood spin-echo sequences, enabling the detection of pericardial thickening on T1-weighted sequences (≥3 mm) . Moreover, during active inflammation, increased gadolinium uptake predicts pericardial edema, which can be assessed and graded using T2-weighted short tau inversion recovery (T2-STIR) sequences. From a clinical perspective, CMR is an essential imaging modality that aids not only in the diagnosis and risk stratification of select patients with AP but also offers the ability to guide IGT and predict the likelihood of disease recurrence (Table 1).3
Table 1: Characteristics of Different Pericardial Pathologies Across Different Imaging Modalities

CMR = cardiac magnetic resonance; CP = constrictive pericarditis; CT = computed tomography; CTP = cardiac tamponade; HU = Hounsfield units; IVC = inferior vena cava; LGE = late gadolinium enhancement; PEff = pericardial effusion; SVC = superior vena cava; T2-STIR = T2-weighted short tau inversion recovery; TTE = transthoracic echocardiography; WMAs = wall motion abnormalities.
Recurrent, Incessant, and Chronic Pericarditis
Recurrent pericarditis (RP) occurs in 20-30% of patients following an initial episode of AP and presents mainly with an inflammatory phenotype, characterized by fever and elevated inflammatory biomarkers.4 IL-1 blockers are recommended for the treatment of this condition, whereas empirical corticosteroids are indicated if a noninflammatory phenotype is encountered.2 CMR is usually required to assist in the diagnosis and prognostication of patients with suspected RP and is useful in monitoring disease activity.4 During acute inflammation, the neovascularized pericardium exhibits contrast enhancement on CMR with edema on T2-STIR and late gadolinium enhancement (LGE). In the chronic phase, inflammation can be assessed by the absence of LGE on CMR and the presence of pericardial thickening and calcifications on cardiac CT.5 CT and CMR can be employed at baseline and then at 6-12 month intervals to guide IGT and assess treatment response.2 In cases of burnt-out and calcific pericarditis, radical pericardiectomy is recommended in experienced, high-volume surgical centers, with cardiac CT playing an important role in presurgical planning (Figure 1).1
Figure 1: Imaging-Guided Therapies for the Continuum of Pericardial Inflammation From an Acute Episode of Pericarditis to End-Stage Refractory Pericarditis or Burnt-Out Calcific CP
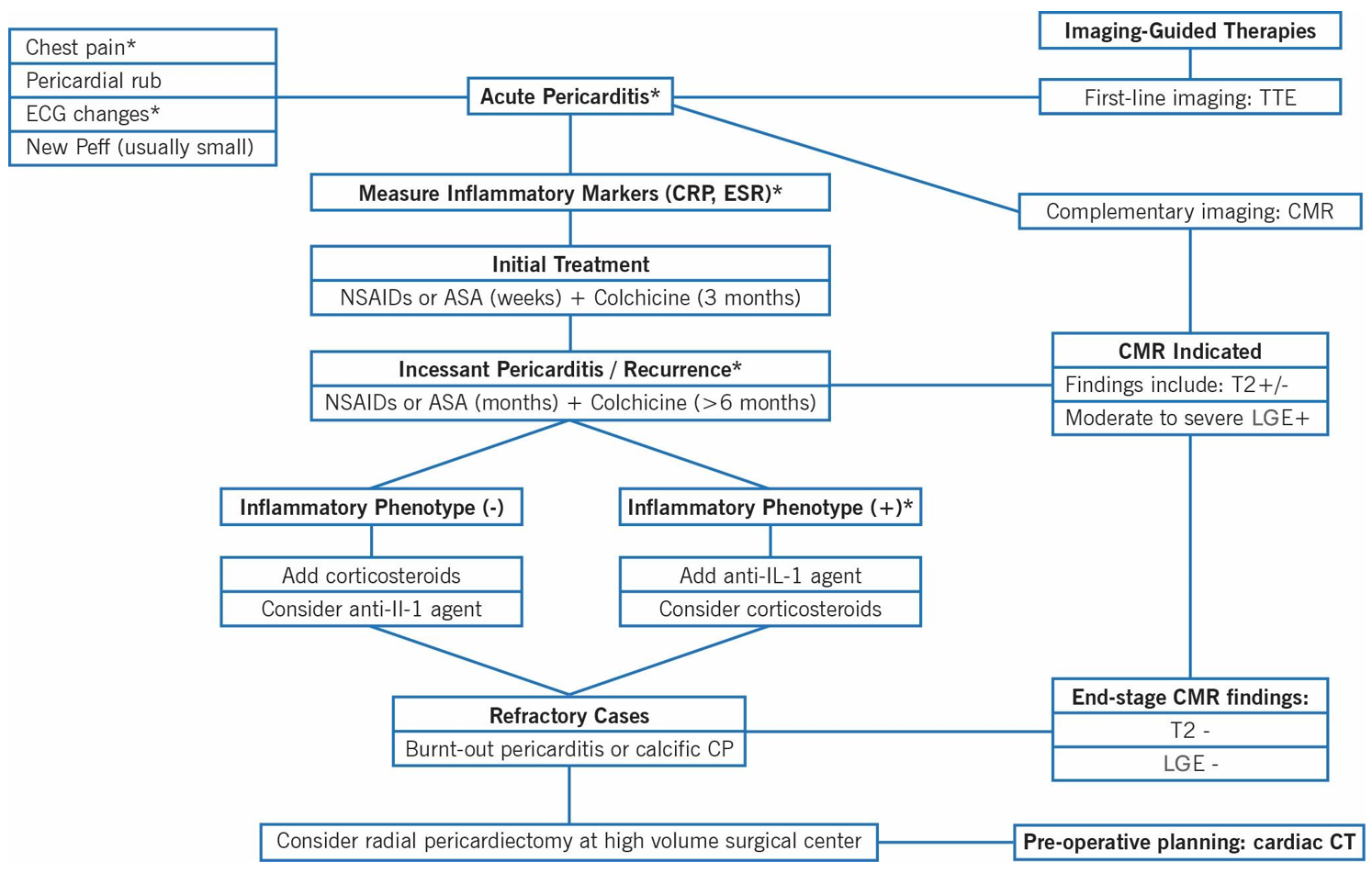
- Chest pain: In AP, is typically sharp and pleuritic, improved by sitting up and leaning forward.
- ECG changes: Include diffuse ST-segment elevations and/or PR-segment depressions.
- AP: At least two of the four criteria fulfilled with duration of symptoms 4-6 weeks. Incessant pericarditis: >4-6 weeks to 3 months without remission. RP: New pericarditis flare after symptom-free interval of >4-6 weeks.
- Inflammatory markers (CRP, ESR): Inflammatory phenotype: high fever, raised CRP level, pleural involvement, neutrophilic leukocytosis with low lymphocyte count and high neutrophil/lymphocyte ratio.
Pericardial Effusion and Cardiac Tamponade
Cardiac tamponade (CTP) is an emergency condition that is usually diagnosed at the bedside using TTE, which allows for rapid and accurate detection, sizing and localization of PEffs, and the evaluation of their hemodynamic impact (Figure 2). It also allows assessment of the feasibility of percutaneous drainage and aids in directly guiding pericardiocentesis.1 The application of cMMI in CTP is usually reserved for cases of diagnostic uncertainty, to rule out potential mimickers, and to guide the drainage of challenging/complex effusions. This is particularly relevant after cardiac surgery when focal intrapericardial hematoma is encountered and may cause local compression. In fact, cardiac CT provides high spatial resolution to perform multiplanar reconstructions, which aids in the localization and sizing of PEffs, and further characterization of fluid by density assessment. Cardiac CT shows value in planning pericardiocentesis in challenging cases in which PEff is small or multiloculated. Additionally, CMR is indicated if there is suspicion of coexisting pericarditis or when pericardial malignant neoplasms/masses are suspected.3 A surgical pericardial window is recommended when a percutaneous approach is not safe or technically feasible, or when recurrences are expected (malignant neoplasms or autoimmune disorders).1
Figure 2: Novel Algorithm for the Diagnosis and Management of PEff
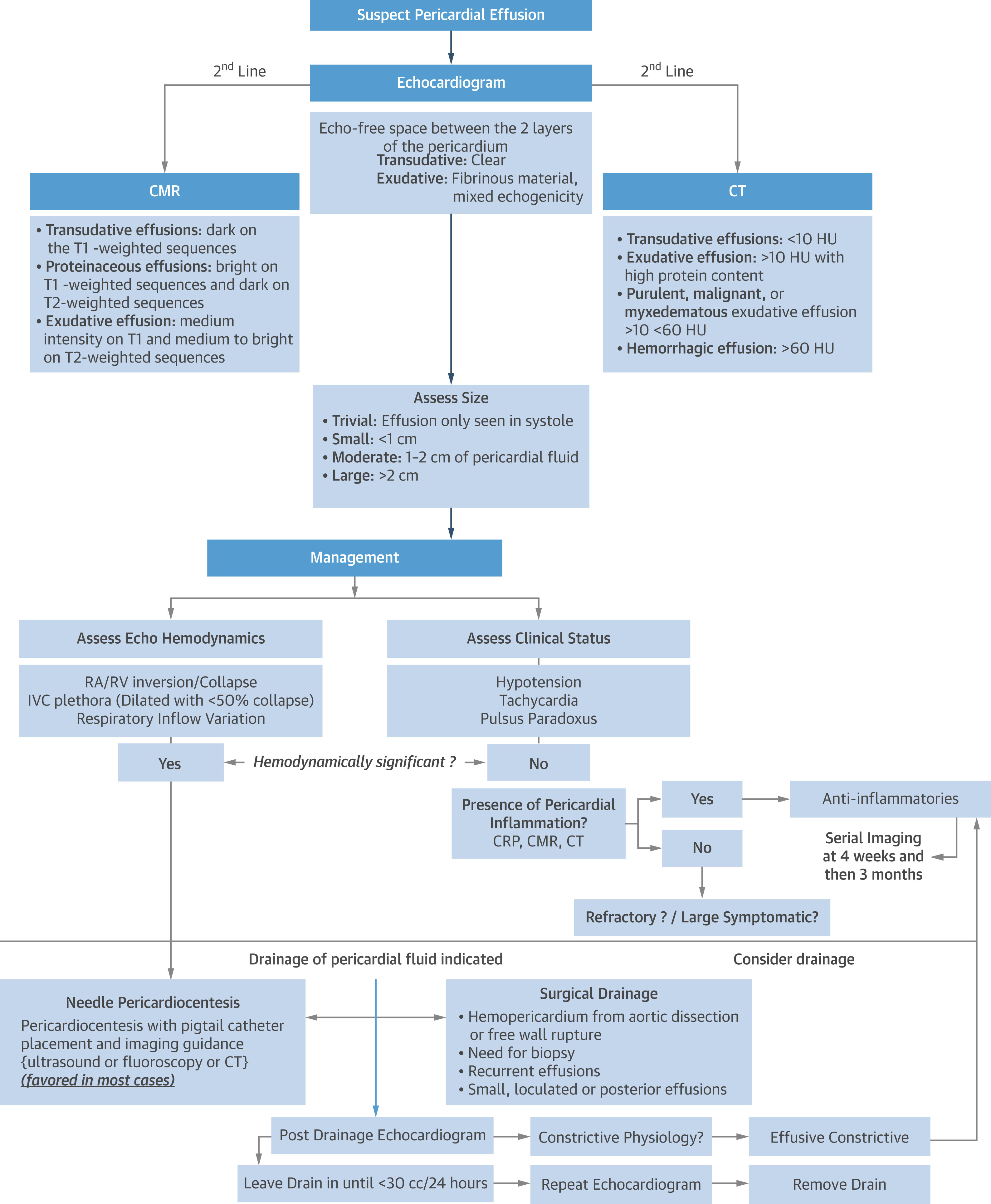
CMR = cardiac magnetic resonance; CRP = C-reactive protein; CT = computed tomography; HU = Hounsfield units; IVC = inferior vena cava; PEff = pericardial effusion; RA = right atrial; RV = right ventricular.
Constrictive Pericarditis
CP lies along the spectrum of pericardial inflammation and thickening, eventually leading to loss of pericardial elasticity and the development of a constrictive physiology. The role of imaging in CP serves to confirm the hemodynamics of CP and to define the presence of pericardial inflammation (Figure 3). TTE, the first-line imaging modality when CP is suspected, offers a high positive predictive value in identifying features of CP.1 Although CMR is a complementary imaging modality, it is used for longitudinal follow-up, allowing for objective assessment of treatment response and disease remission by offering qualitative and quantitative assessment of LGE. CMR has also been shown to predict responses to anti-inflammatory therapies.3,6 Cardiac CT is a valuable imaging modality used in preoperative planning for pericardiectomy, offering detailed evaluation of pericardial thickness and the presence of calcifications in cases of CP.1 Anti-inflammatory therapies, including corticosteroids or anti–IL-1 agents, in combination with colchicine, may be instituted for a period of ≥8-12 weeks before clinical and imaging reevaluation.7 However, anti-inflammatory therapies are not indicated in the setting of CP without evidence of active inflammation on CMR. Symptomatic CP should be managed with a comprehensive surgical pericardiectomy, which should ideally be performed by a surgeon with expertise in pericardial surgery and should not be done during active inflammation. A traditional partial pericardiectomy is inadequate because it is associated with lower long-term survival rates and less improvement in echocardiographic outcomes.1
Figure 3: A Multimodal Approach to the Noninvasive Diagnosis of CP
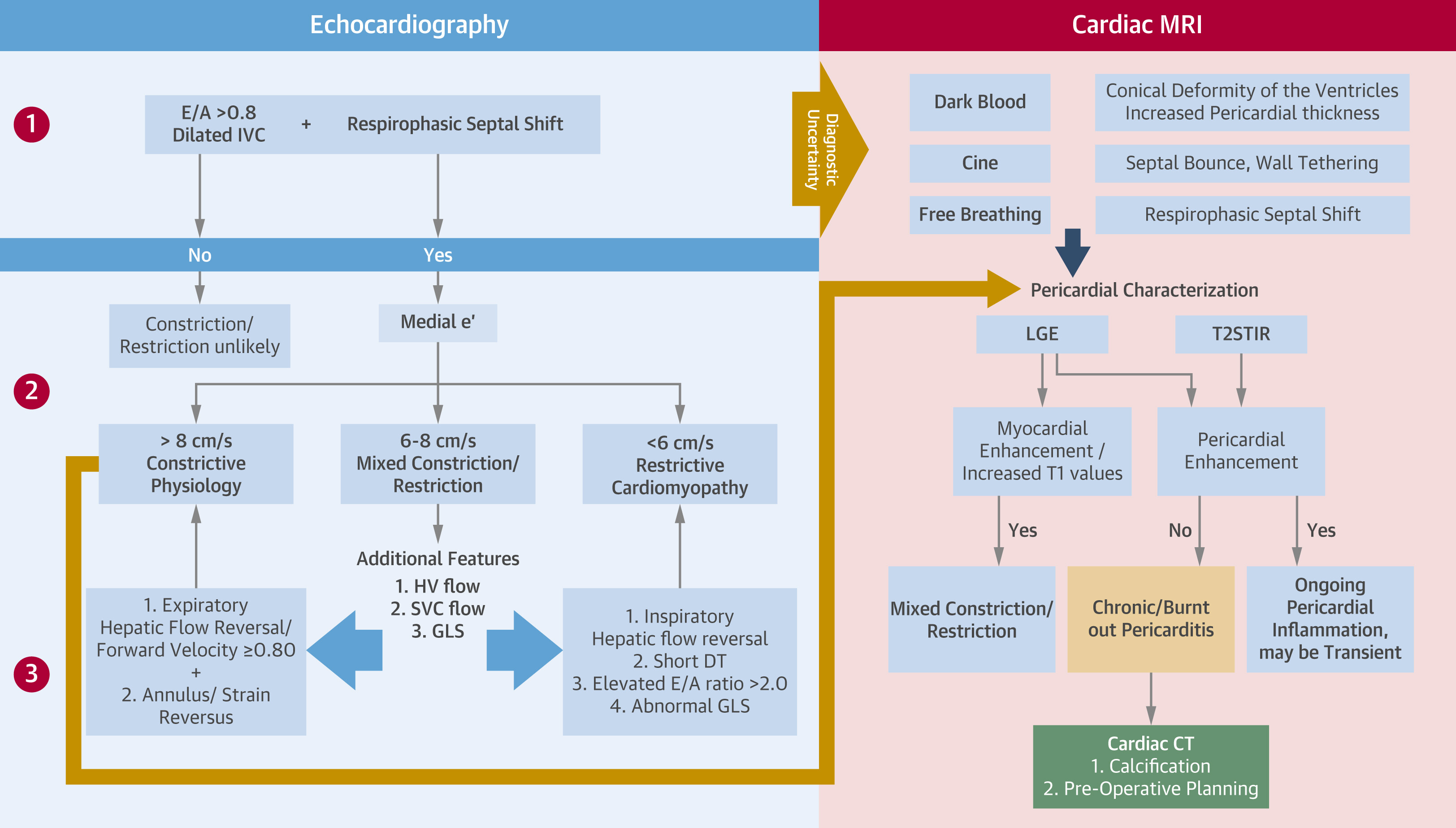
CP = constrictive pericarditis; CT = computed tomography; DT = deceleration time; GLS = global longitudinal strain; HV = hepatic vein; IVC = inferior vena cava; LGE = late gadolinium enhancement; LVGLS = left ventricular global longitudinal strain; MRI = magnetic resonance imaging; SVC = superior vena cava; T2STIR = T2-weighted short tau inversion recovery.
Conclusion
The spectrum of pericardial disorders encompasses pericardial inflammation, effusion, constriction, and masses (Table 1). Integrated cMMI is pivotal in the comprehensive evaluation of these conditions and, with the rise of the novel IL-1 blockers, IGT is now an integral component of the diagnosis, management, risk stratification, and monitoring of treatment response.
References
- Klein AL, Wang TKM, Cremer PC, et al. Pericardial diseases: international position statement on new concepts and advances in multimodality cardiac imaging. JACC Cardiovasc Imaging 2024;17:937-88.
- Klein AL, Imazio M, Cremer P, et al.; RHAPSODY Investigators. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med 2021;384:31-41.
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2017;70:1647-72.
- Imazio M, Mardigyan V, Andreis A, Franchin L, de Biasio M, Collini V. New developments in the management of recurrent pericarditis. Can J Cardiol 2023;39:1103-10.
- Conte E, Agalbato C, Lauri G, et al. Cardiac MRI after first episode of acute pericarditis: a pilot study for better identification of high-risk patients. Int J Cardiol 2022;354:63-7.
- Gastl M, Sokolska JM, Polacin M, et al. Parametric mapping CMR for the measurement of inflammatory reactions of the pericardium. Open Heart 2022;9:[ePub ahead of print].
- Chetrit M, Xu B, Kwon DH, et al. Imaging-guided therapies for pericardial diseases. JACC Cardiovasc Imaging 2020;13:1422-37.
Clinical Topics: Noninvasive Imaging, Pericardial Disease, Echocardiography/Ultrasound, Magnetic Resonance Imaging
Keywords: Pericarditis, Echocardiography, Computed Tomography, Magnetic Resonance Imaging, Diagnostic Imaging, Cardiac Imaging Techniques
