Preoperative Cessation of SGLT2i
Quick Takes
- Sodium-glucose co-transporter 2 (SGLT2)-inhibitors are increasingly being prescribed by cardiovascular clinical teams for patients with heart failure with and without diabetes mellitus.
- The cardiovascular teams should be aware of recent Food and Drug Administration (FDA) advisory warnings for increased incidence of euglycemic diabetic ketoacidosis when SGLT2-inhibitors medications are continued prior to non-cardiac surgeries.
- These warnings have stated that continuing SLGT2-inhibitors preoperatively can lead to poor patient outcomes and increased hospital length of stay.
- When caring for patients being referred for surgery it is important to advise patients to stop their SGLT2-inhibitors 3-4 days prior to surgery to minimize the risk of postoperative ketoacidosis and urinary tract infections.
Euglycemic diabetic ketoacidosis (EDKA) is an uncommon but life-threatening diagnosis and must be considered in postoperative patients who have been on SGLT2-inhibitors. EDKA in this setting has been reported to occur any time during the course of medication use.
It should be considered when a patient has:
- An anion gap metabolic acidosis and pH <7.3
- Elevated ketones in the blood or urine
- A blood glucose <200
Given the clinical overlap with starvation ketoacidosis, it is a difficult diagnosis to make. The mechanism of both is similar, except that in EDKA, the patient has diabetes mellitus and has been taking a SLGT2-inhibitor.
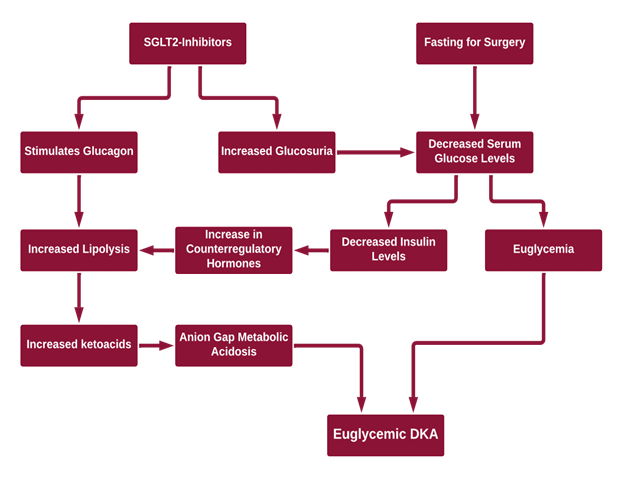
The mechanism (Figure 1) by which this phenomenon is thought to occur is through an increased glucagon:insulin ratio. SGLT2-inhibitors can cause:1-3
- Increasing urinary excretion of glucose by blocking reabsorption of glucose at the proximal convoluted tubule
- Lower insulin levels needed to maintain fasting glucose levels which can lead to ketosis
- Increased glucagon
- Hypovolemia due to glucosuria
- An increase in counter-regulatory hormones which lead to lipolysis and ketosis
- Suppressed removal of beta-hydroxybutyrate and acetoacetate by the kidneys
Figure 1: Proposed Mechanisms of EDKA
The symptoms of EDKA include anorexia, nausea, vomiting, and dyspnea. The treatment is similar to that of DKA except patients oftentimes need a dextrose drip to allow for high enough doses of insulin to suppress ketone formation and close the anion gap. The patient should be discharged without their SGLT2-inhibitor.1
The FDA released multiple warnings4 related to the elevated risk of DKA. The recommendation is for canagliflozin, dapagliflozin, and empagliflozin to be discontinued 3 days before scheduled surgery and ertugliflozin should be stopped at least 4 days prior to surgery. This differs from other diabetic medications which are typically held the day of surgery. With the increase in SLGT2-inhibitors being prescribed for both diabetes and heart failure, it is crucial that providers are aware of this preoperative hold parameter and recognize patients at risk for EDKA.
References
- Plewa MC, Bryant M, King-Thiele R. Euglycemic Diabetic Ketoacidosis (StatPearls [Internet]). 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK554570/. Accessed 09/01/2022.
- Lam DWH. Euglycemic Diabetic Ketoacidosis in the Modern Era (youtube.com) 2018. Available at: https://www.youtube.com/watch?v=-UNimRawMHg. Accessed 09/01/2022.
- Wang KM, Isom RT. SGLT2-inhibitor induced euglycemic diabetic ketoacidosis: a case report." Kidney Med 2020;2:218-221.
- FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections (fda.gov). 2022. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious. Accessed 09/01/2022.
Clinical Topics: Dyslipidemia, Heart Failure and Cardiomyopathies, Lipid Metabolism, Acute Heart Failure, Geriatric Cardiology
Keywords: Sodium-Glucose Transporter 2, Diabetic Ketoacidosis, Canagliflozin, Blood Glucose, 3-Hydroxybutyric Acid, Acetoacetates, Glucagon, Acid-Base Equilibrium, Hypovolemia, Lipolysis, Patient Discharge, Nausea, Vomiting, Dyspnea, Heart Failure, Insulins, Kidney, Sodium-Glucose Transporter 2 Inhibitors
< Back to Listings