Combination Chemotherapy/Immunotherapy Regimens in Patients With Breast Cancer
Quick Takes
- The KEYNOTE-522 phase 3 trial demonstrated significant improvements in event-free survival and pathological complete response in previously untreated stage II or III triple-negative breast cancer for patients treated with pembrolizumab and chemotherapy compared with chemotherapy alone, particularly in HER2-negative breast cancer.
- Combination therapy with pembrolizumab and chemotherapy raised concerns regarding myocarditis, with higher incidence rates observed compared with chemotherapy alone, highlighting the need for close monitoring and further investigation of cardiovascular risks associated with these treatments.
- European Society of Cardiology guidelines recommend pretreatment risk assessment and ongoing surveillance using echocardiography and biomarkers for high-risk individuals undergoing immunochemotherapy, emphasizing the importance of early detection and management of myocarditis and cancer therapy–related cardiac dysfunction in patients with breast cancer.
Figure 1: KEYNOTE-522 Study Cohorts and Outcomesa
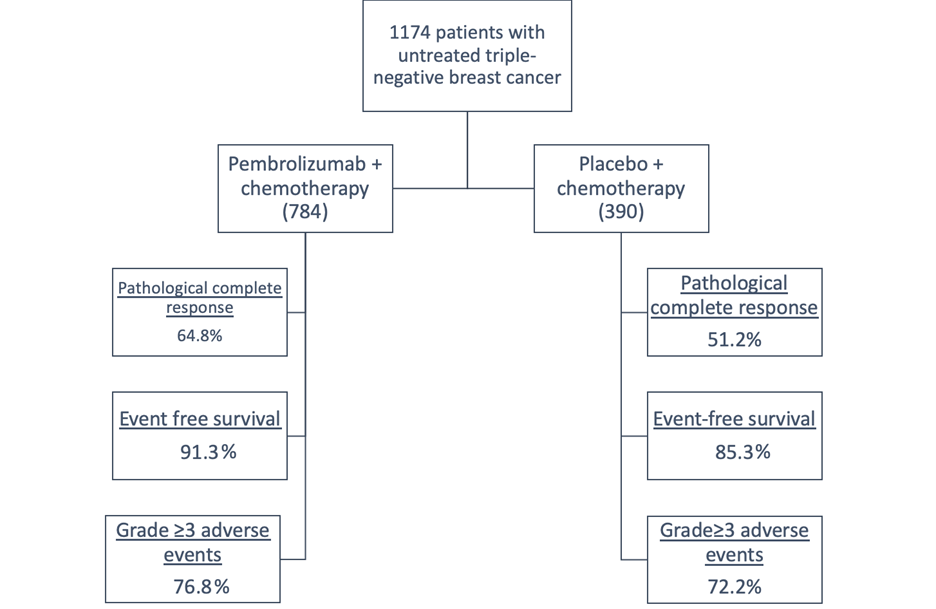
aAdapted with permission from Schmid P, Cortes J, Pusztai L, et al.; KEYNOTE-522 Investigators. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810-21.
Key Oncology Trial for Immunochemotherapy in Breast Cancer Patients
One pivotal study, the phase 3 trial known as KEYNOTE-522,1 focused on previously untreated stage II or stage III triple-negative breast cancer patients. These individuals were randomly assigned to receive pembrolizumab in combination with chemotherapy, which was found to potentially enhance endogenous anticancer immunity by increasing the release of tumor-specific antigens.
Patients were divided into two groups: a placebo chemotherapy group and a pembrolizumab-chemotherapy group. The former received a placebo alongside paclitaxel and carboplatin, followed by four additional cycles of placebo or doxorubicin-cyclophosphamide/epirubicin-cyclophosphamide. In contrast, the latter received pembrolizumab every 3 weeks in conjunction with paclitaxel and carboplatin, followed by four additional cycles of pembrolizumab or doxorubicin-cyclophosphamide/epirubicin-cyclophosphamide.
Remarkably, this effect was observed in patients with human epidermal growth factor receptor 2 (HER2)-negative breast cancer. The results showed a 6% improvement in event-free survival and 13% improvement in pathological complete response at the time of surgery.
Concern for Adverse Cardiovascular Effects of Immunochemotherapy
Myocarditis is a rare (1%) but life-threatening adverse effect of immune checkpoint inhibitors with up to 50% mortality. In the pembrolizumab-chemotherapy group, myocarditis was observed in 3/784 (0.4%) of patients, whereas in the placebo group, none of the patient's experienced myocarditis.1 This incidence is similar to patients with Hodgkin lymphoma who receive combination immune checkpoint inhibitors and chemotherapy.
Combination therapy with anthracyclines and immune checkpoint inhibitors have been proposed to maximize cancer therapy; however, more information is needed regarding adverse cardiovascular events. In mouse model studies, use of high-dose anthracycline decreased the concentration of programmed death-1 (PD1) ligands, thus possibly contributing to increased susceptivity to immune-related adverse effects of immune checkpoint inhibitor therapy.2
In the KEYNOTE-087 trial, in patients with relapsed/refractory classical Hodgkin lymphoma who had received prior treatment with anthracycline chemotherapy, the use of pembrolizumab was associated with a 1% incidence of myocarditis. Median time of onset for myocarditis is 30 days after initiation of therapy.3 Risk factors include combination immune checkpoint inhibitor therapy (anti-cytotoxic T-lymphocyte associated protein 4 in conjunction with anti-PD1 therapy) with up to a 4.7-fold risk compared with single therapy.4 Other theoretical risk includes previous myocardial injury, pre-existing autoimmune disease, and genetic predisposition.5
In the SWOG S1826 trial, combination nivolumab and doxorubicin, vinblastine, and dacarbazine (AVD) for Hodgkin lymphoma has been studied.6 There were no cardiac events reported. In one smaller study of 30 patients with classic Hodgkin lymphoma who received pembrolizumab with addition of AVD therapy with a total dose of 300 mg/m2 of doxorubicin, one patient developed symptoms of heart failure and another developed cardiomyopathy, but no incidence of myocarditis was reported.7
These findings underscore a potential risk associated with the addition of an immune checkpoint inhibitor with anthracycline treatment, highlighting a possible increased susceptibility to cardiovascular complications in this context. The long-term cardiovascular effects of these treatments are unknown and require vigilance from the cardiology, oncology, and cardio-oncology communities.
It is important to note that findings may be specific to the timing of the addition of immune checkpoint inhibitors to anthracycline-based chemotherapy, either before, simultaneously, or after chemotherapy administration.
Surveillance for Myocarditis and Cancer Therapy–Related Cardiac Dysfunction in Immunochemotherapy
Managing such patients presents unique challenges. The European Society of Cardiology (ESC) guidelines offer some direction on the surveillance of these high-risk individuals, emphasizing the role of echocardiography and biomarkers like naturetic peptides (NP) such as N-terminal pro–B-type natriuretic peptide and cardiac troponin (cTn) I or T in monitoring cardiac health on each of these two types of treatment separately. Patients should be risk assessed pretreatment. For patients on anthracycline therapy, a transthoracic echocardiogram should be obtained at baseline and at 1-year post treatment. For high and very high-risk patients on anthracycline therapy, a transthoracic echocardiogram is recommended at every other cycle through treatment and at 3- months and 12-months post treatment, alongside biomarkers (cTn/NP) at every cycle and at 3-months and 12-months post treatment. For high-risk patients on immunotherapy, transthoracic echocardiogram and biomarkers (cTn/NP) are indicated at baseline and biomarkers (cTn) may be considered with every cycle for the first four cycles and then periodically. There are no specific guidelines in utilization of biomarkers and echocardiography for combination therapy, but more frequent monitoring and careful symptom monitoring should be considered. This underscores the importance of pretreatment cardiac risk assessment and optimization prior to starting immunochemotherapy.
Surveillance for cardiovascular complications, particularly cancer therapy–related cardiac dysfunction and myocarditis in patients with breast cancer undergoing immunochemotherapy, represents an evolving field with several key considerations. While immunotherapy holds promise in breast cancer treatment, its widespread applicability is still emerging, especially as new studies and genetic cancer analyses shape its future role.
Future Directions
It is important to emphasize the paucity of data in this area of cardio-oncology. The precise mechanisms by which the prior use of anthracyclines might exacerbate the risk of immune checkpoint inhibitor–related cardiotoxicity remain poorly understood. To address this knowledge gap and tailor treatments effectively, real-world data is essential. Such data can shed light on the extent of risk and help refine treatment strategies for individual patients.
Administering immune checkpoint inhibitors to patients receiving doxorubicin and anti-HER2 therapy as part of their chemotherapy regimen likely presents a substantial risk of cardiotoxicity. This risk may not only carry the potential for increased mortality and morbidity, but also poses limitations on the continuation of anticancer treatment in these patients.
For those receiving multiple treatments with potentially cardiotoxic agents, close surveillance is imperative. Monitoring using serum biomarkers and regular echocardiography may become a necessity to ensure the safe administration of chemotherapy and promptly detect and manage any adverse cardiac events.
In summary, as the landscape of immunochemotherapy in breast cancer evolves, thorough surveillance and data-driven strategies are vital components of patient care in this complex and dynamic field. It is important to consider both oncologists' perspectives and broader viewpoints on the low incidence of myocarditis in treated patients. Since most patients do not develop this complication and achieve better survival and remission rates, efforts should focus on identifying the subset of patients who are more prone to develop myocarditis and the ideal biomarkers that can detect those at higher risk for myocarditis before starting chemotherapy.
Table 1: Key Trials of Immunochemotherapy in Breast Cancer Patients
IMpassion050: A Study To Evaluate the Efficacy and Safety Of Atezolizumab in Combination With Neoadjuvant Doxorubicin + Cyclophosphamide Followed By Paclitaxel + Trastuzumab + Pertuzumab In Early HER2-Positive Breast Cancer8
|
IMpassion130: A Study of Atezolizumab in Combination With Nab-Paclitaxel Compared With Placebo With Nab-Paclitaxel for Participants With Previously Untreated Metastatic Triple-Negative Breast Cancer9,10
|
IMpassion031: A Study to Investigate Atezolizumab and Chemotherapy Compared With Placebo and Chemotherapy in the Neoadjuvant Setting in Participants With Early Stage Triple Negative Breast Cancer9
|
KEYNOTE-119: Study of Single Agent Pembrolizumab Versus Single Agent Chemotherapy for Metastatic Triple Negative Breast Cancer11
|
KEYNOTE-355: Study of Pembrolizumab Plus Chemotherapy vs. Placebo Plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple Negative Breast Cancer12
|
KEYNOTE-522: Pembrolizumab for Early Triple-Negative Breast Cancer13
|
GeparNUEVO: A Randomized Phase II Study Investigating Durvalumab in Addition to an Anthracycline Taxane-Based Neoadjuvant Therapy in Early Triple-Negative Breast Cancer14
|
Reference: ClinicalTrials.gov
References
- Schmid P, Cortes J, Dent R. Pembrolizumab in early triple-negative breast cancer. Reply. N Engl J Med 2022;386:1771-2.
- Korste S, Settelmeier S, Michel L, et al. Anthracycline therapy modifies immune checkpoint signaling in the heart. Int J Mol Sci 2023;24:6052.
- Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 2019;134:1144-53.
- Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579-89.
- Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755-64.
- Herrera AF, LeBlanc ML, Castellino SM, et al. SWOG S1826, a randomized study of nivolumab(N)-AVD versus brentuximab vedotin (BV)-AVD in advanced stage (AS) classic Hodgkin lymphoma (HL). Abstract LBA4. Presented at the 2023 American Society of Clinical Oncology Annual Meeting, June 4, 2023; Chicago, Illinois.
- Lynch RC, Ujjani CS, Poh C, et al. Concurrent pembrolizumab with AVD for untreated classic Hodgkin lymphoma. Blood 2023;141:2576-86.
- Huober J, Barrios CH, Niikura N, et al.; IMpassion050 Trial Investigators. Atezolizumab with neoadjuvant anti-human epidermal growth factor receptor 2 therapy and chemotherapy in human epidermal growth factor receptor 2-positive early breast cancer: primary results of the randomized phase III IMpassion050 trial. J Clin Oncol 2022;40:2946-56.
- Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 2020;396:1090-100.
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108-21.
- Winer EP, Lipatov O, Im SA, et al.; KEYNOTE-119 investigators. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:499-511.
- Cortes J, Rugo HS, Cescon DW, et al.; KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med 2022;387:217-26.
- Schmid P, Cortes J, Pusztai L, et al.; KEYNOTE-522 Investigators. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810-21.
- Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 2019;30:1279-88.
Clinical Topics: Cardio-Oncology
Keywords: Cardio-oncology, Immunotherapy, Induction Chemotherapy, Breast Neoplasms
