I Am an Athlete: Will This Statin Affect My Performance?
Quick Takes
- Athletes are more prone to the development of statin-associated muscle symptoms (SAMS); however, there is inconclusive evidence to support a pathophysiologic effect of statins on muscle weakness or reduced aerobic capacity.
- Clinicians should consider a stepwise approach to prevent the development of SAMS, discussing the risks and benefits of statin therapy with the patient, documenting pre-existing musculoskeletal symptoms, and identifying SAMS risk factors before initiating a nonlipophilic statin such as rosuvastatin.
- A stepwise approach to the management of SAMS when they occur is suggested, and many athletes will tolerate a subsequent trial of statins or an alternative lipid-lowering therapy.
Case Presentation
A 59-year-old woman with newly diagnosed nonobstructive coronary artery disease (CAD) presents to the sports cardiology clinic. She is a long-distance runner who has participated in numerous marathons over the previous 20 years. She had been in her usual state of health up until 2 months before presentation, when she developed anginal symptoms and decreased exercise capacity. Invasive coronary angiography demonstrated nonobstructive CAD. She was initiated on a beta-blocker to treat suspected microvascular ischemia, with substantial improvement in her symptoms and exercise capacity. She has concerns regarding starting a statin given the potential for statin-associated muscle symptoms (SAMS).
Definitions
The most frequent statin-associated adverse effects are SAMS, which occur in 5-20% of patients in observational studies.1 Interestingly, randomized data suggest that >90% of reported muscle symptoms are not statin related.2 The umbrella term SAMS incorporates: 1) subjective myalgias, cramps, or a sense of weakness with creatine kinase (CK) level within the reference range; 2) myositis/myopathy, defined as muscle symptoms or weakness with CK level greater than the upper limit of normal (ULN); 3) rhabdomyolysis, defined as CK level >10 times the ULN and renal injury; and 4) statin-associated autoimmune myopathy, defined as necrotizing myopathy with the presence of hydroxy-methyl-glutaryl coenzyme A reductase antibodies and incomplete resolution on statin discontinuation. Myalgias account for most SAMS, whereas the other clinical entities are rare.
Are Athletes More Prone to SAMS?
Factors that predispose people to SAMS in the general population include demographic factors, comorbidities, and high-risk medications (Table 1).3 SAMS are more common among athletes and physically active individuals. Among 832 individuals who reported SAMS on the PRIMO (Prediction of Muscular Risk in Observational conditions) survey, the incidence of SAMS was 10.8% in those who participated in leisure activity and 14.7% in those who participated in vigorous sport.4
Table 1: Factors That Predispose to Statin-Associated Muscle Symptoms
| Demographics | Comorbidities | Medications (CYP3A4 Interactions) |
| Older age | HIV | Antimicrobials (ketoconazole, clarithromycin, HIV protease inhibitors) |
| Female sex | Renal disease | |
| Lower BMI | Liver disease | Calcium channel blockers |
| Asian ethnicity | Thyroid disease | Fibrates |
| Athlete | Alcohol excess | Other (amiodarone, ranolazine) |
BMI = body mass index; CYP3A4 = cytochrome P450 3A4.
Does Statin Use Predispose Athletes to Muscle Injury, Weakness, or Reduced Aerobic Capacity?
The results of earlier studies suggested a link between statins and both reduced aerobic capacity and objective muscle weakness, particularly among older individuals. However, the STOMP (Effect of Statins on Skeletal Muscle Function and Performance) randomized placebo-controlled trial assessed 420 statin-naïve, healthy individuals followed for 6 months. Atorvastatin 80 mg, compared with placebo, resulted in more subjective myalgias and higher CK levels but did not reduce muscle strength or exercise performance.5 In contrast, 100 athletes who participated in the 4Days Marches (DE 4DAAGSE): The Walk of the World in Nijmegen, The Netherlands did not show any statin-related differences. After walking 30-50 km per day for 4 days, statin therapy did not worsen exercise-induced muscle symptoms, CK levels increased in all groups, and statin therapy did not augment exercise-induced muscle injury.6
Potential Mechanisms of Exercise-Associated Damage
Multiple mechanisms have been implicated in exercise-associated muscle damage, including the depletion of intramuscular coenzyme Q10 levels, disruption of the ubiquitin proteasome pathway altering the balance between cell degradation and repair, and impaired sarcoplasmic reticulum calcium cycling.7 With exercise, these mechanisms reduce adenosine triphosphate availability, resulting in myocyte membrane permeability and fiber damage. Genetic factors and vitamin D deficiency increase susceptibility to SAMS, although available evidence does not support routine supplementation with vitamin D or coenzyme Q10.5
Worst Offending Agents
Lipophilic statins, such as atorvastatin, simvastatin, and lovastatin, have been associated with the highest risk of statin-related myopathy, partially because of enhanced muscle penetration.8 These medications are also highly metabolized by hepatic cytochrome P450 3A4(CYP3A4) isoenzymes, so certain antimicrobials, calcium channel blockers, and fibrates—all of which interfere with CYP3A4 function—increase the risk of myopathy and rhabdomyolysis.
The "Nocebo" Effect and Tolerating Subsequent Trials
Substantially higher rates of SAMS have been reported in the results of observational studies than in randomized controlled trials. This difference has been attributed primarily to the "nocebo" effect (i.e., caused by negative expectations). In the SAMSON (Self-Assessment Method for Statin Side-effects Or Nocebo) trial, >50% of individuals who stopped a statin because of SAMS could tolerate one when reintroduced.9,10 Furthermore, the DESIFOR (DEtermining Statin Intolerance For Rosuvastatin) trial data showed that over two-thirds of statin-intolerant individuals could tolerate unblinded rosuvastatin use after participating in the n-of-1 study.11
Prevention of SAMS
Athletes tend to be more intolerant of statins; however, individuals at high risk who stop statin therapy have a markedly increased risk of cardiovascular (CV) events.3 It is therefore crucial to take a stepwise approach to optimize both the prevention of SAMS and its management when it develops (Figure 1).
Figure 1: Stepwise Approach to Prevention of Statin-Associated Muscle Symptoms
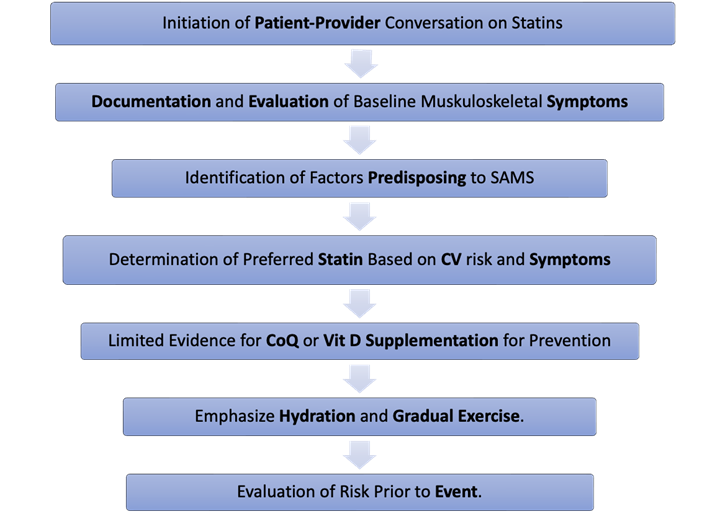
CoQ = coenzyme Q10; CV = cardiovascular; SAMS = statin-associated muscle symptoms; Vit D = vitamin D.
First, clinicians should take time to discuss the potential benefits and risks of initiating therapy and to identify patient concerns and preferences. Second, they should document a comprehensive evaluation of musculoskeletal symptoms because such symptoms are common at baseline. The European Society of Cardiology (ESC) guidelines recommend obtaining a baseline CK level on the basis of consensus rather than evidence, whereas the American College of Cardiology/American Heart Association (ACC/AHA) guidelines do not. Third, clinicians should identify predisposing factors for SAMS as outlined in Table 1. Fourth, they should prescribe a statin intensity suited to the patient's CV risk. Practically speaking, the authors typically use rosuvastatin for individuals at high risk and pravastatin for those at lower risk who have significant concerns about SAMS. Because of its effective lowering of low-density lipoprotein (LDL) and risk of SAMS, low-dose rosuvastatin, with or without ezetimibe, can be considered in patients concerned about SAMS. Initiation of therapy may be deferred until after a major event. Fifth, clinicians should not routinely recommend the use of coenzyme Q10 or vitamin D supplementation in the absence of deficiency to prevent SAMS. Sixth, they should emphasize the importance of hydration and graduated exercise increases (intensity and duration) in preventing rhabdomyolysis. Seventh, in exceptional circumstances, especially if the patient has relatively low CV risk (<5% 10-year atherosclerotic cardiovascular disease [ASCVD] risk) and is particularly concerned about SAMS, the authors suggest holding the drug for 1-2 days before a major event.
Management of SAMS
With clinical evaluation and CK level measurement, clinicians can identify whether the clinical syndrome is consistent with myalgia, weakness, myositis, or rhabdomyolysis. For milder symptoms, the SAMS clinical index tool can objectively assess the likelihood that muscle symptoms were caused by statin therapy (more likely if bilateral proximal muscles are involved, development within weeks to months of starting a statin, and resolution after the statin is discontinued). Second, if the CK level is less than four times the ULN, the statin should be stopped and clinicians should follow the flow diagram (Figure 2) when introducing a new medication. If the CK level is more than four times the ULN, the statin should be stopped for 6 weeks and the patient monitored for normalization of symptoms and CK levels before the reintroduction of a low-dose, potent statin or alternate-day dosing.
Figure 2: Suggested Algorithm for Statin-Associated Muscle Symptoms12
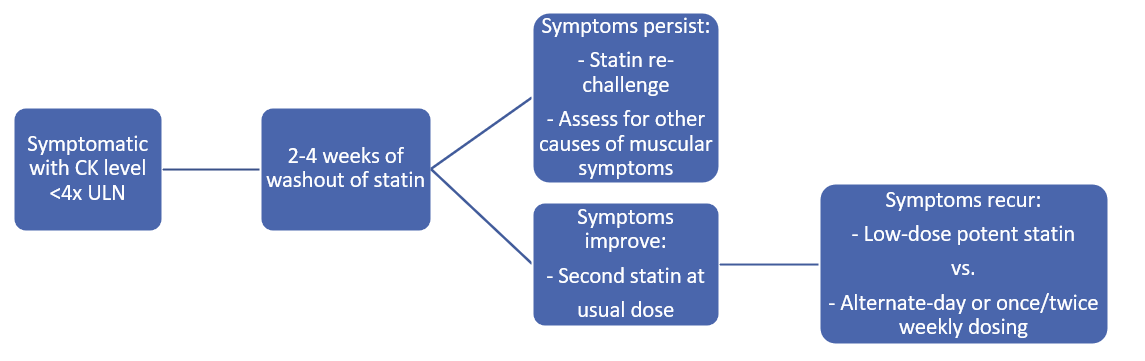
CK = creatine kinase; ULN = upper limit of normal.
Patients with rhabdomyolysis (CK level >10 times the ULN) require immediate attention and may need to discontinue statin use indefinitely; however, reversible causes, such as dehydration or interacting drugs, should be sought. Clinicians should not reintroduce a statin if statin-associated autoimmune myopathy is diagnosed. They should use alternative lipid-lowering therapy, such as ezetimibe, bempedoic acid, and proprotein convertase subtilisin/kexin type 9 inhibitors, to lower LDL cholesterol levels and reduce ASCVD risk in patients who cannot take statins.
References
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285-e350.
- Katsiki N, Mikhailidis DP, Bajraktari G, et al.; International Lipid Expert Panel (ILEP). Statin therapy in athletes and patients performing regular intense exercise - position paper from the International Lipid Expert Panel (ILEP). Pharmacol Res 2020;155:[ePub ahead of print].
- Cholesterol Treatment Trialists' Collaboration. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials. Lancet 2022;400:832-45.
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005;19:403-14.
- Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation 2013;127:96-103.
- Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev 2012;40:188-94.
- Allard NAE, Janssen L, Lagerwaard B, et al. Prolonged moderate-intensity exercise does not increase muscle injury markers in symptomatic or asymptomatic statin users. J Am Coll Cardiol 2023;81:1353-64.
- Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res 2019;124:328-50.
- Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet 2017;389:2473-81.
- Howard JP, Wood FA, Finegold JA, et al. Side effect patterns in a crossover trial of statin, placebo, and no treatment. J Am Coll Cardiol 2021;78:1210-22.
- Miedema MD, Gamam A, Garberich R, White S, Benson G. A double-blinded randomized n-of-1 trial to facilitate tolerance of unblinded rosuvastatin: the DESIFOR pilot trial. JACC Adv 2023;2:[ePub ahead of print].
- Mach F, Baigent C, Catapano AL, et al.; ESC Scientific Document Group. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111-88.
Clinical Topics: Diabetes and Cardiometabolic Disease, Dyslipidemia, Sports and Exercise Cardiology, Atherosclerotic Disease (CAD/PAD), Nonstatins, Novel Agents, Statins, Cardiovascular Care Team, Stable Ischemic Heart Disease, Prevention, Vascular Medicine
Keywords: Athletes, Dyslipidemias, Dyslipidemia, Long Term Adverse Effects, Coronary Artery Disease, Hydroxymethylglutaryl-CoA Reductase Inhibitors
