The Utility of CAC in Efficient Randomized Control Trial Design
Quick Takes
- New drugs aimed at primary prevention of cardiovascular disease are costly to evaluate; a recent study suggests that using participants' coronary artery calcium (CAC) score as entry criterion in future randomized controlled trials can increase study efficiency, and thereby decrease overall expenditure in phase III drug evaluation.
- Selective use of CAC scores can be helpful for increasing value in drug allocation in primary prevention.
Introduction
In 2017, the Food and Drug Administration (FDA) approved twice the number of drugs it did 10 years before.1 While the ever-increasing volume of new therapies creates opportunities to advance care, it also presents a challenge to effectively evaluate and allocate them. A recent study evaluated the utility of the coronary artery calcium (CAC) score in improving the efficiency of randomized control trials (RCT) in cardiovascular primary prevention.2
To address this research question, the authors analyzed data from a large primary prevention cohort and projected costs for the creation and implementation of a RCT that would evaluate a hypothetical novel therapy to be used on top of statin therapy.
Study Methodology
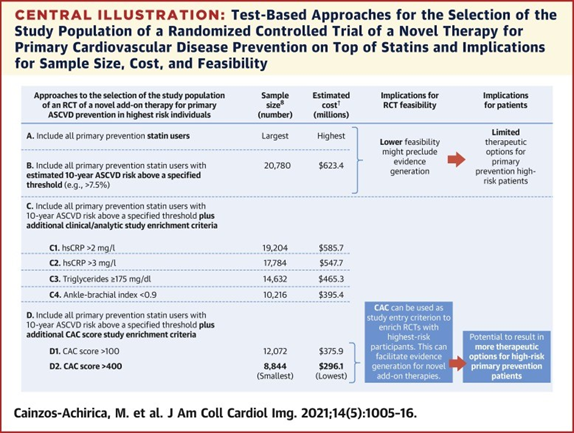
Data from the MESA (Multi-Ethnic Study of Atherosclerosis) cohort was utilized in this analysis of statin-naïve patients. Of the 6,814 patients in the MESA cohort, the inclusion and exclusion criteria were met by 5,777.
For their outcomes, the hypothetical RCT implemented the FDA guidance for pivotal cardiovascular outcome trials and defined an expanded 5-point major adverse cardiovascular event including myocardial infarction, definite angina and probable angina followed by revascularization, stroke, resuscitated cardiac arrest, and cardiovascular disease (CVD) death.3,4
The authors stratified the cohort into four subpopulations based on estimated atherosclerotic cardiovascular disease (ASCVD) risk: ≥7.5%, ≥10%, ≥15%, and ≥20%. Separately, they analyzed the prevalence of two CAC score ranges in each subpopulation: >100 and >400. The number needed to screen (NNS) to identify one participant in each CAC range was also derived for each stratum of ASCVD risk. Of the 5,777 MESA subjects included, 3,075 had an estimated 10-year ASCVD risk of ≥7.5%.
MESA has followed participants for more than 17 years, and it includes data on CVD events within that time frame.3 The authors used the observed 5-year incidence of CVD events and applied that as a background risk in their population. A relative risk reduction (RRR) of 28% was assumed with high-intensity statin therapy,5-7 and the expected 5-year incidence with statin treatment was projected for each subpopulation. Subsequently, the hypothetical therapy being studied was assumed as having either a 15% or 25% RRR on top of statin therapy, and subsequent 5-year incidences were projected.
The authors took this set of projections by subpopulation and estimated the sample size needed for their hypothetical primary prevention phase III RCT. Entry criteria for participants was based on combining increasing ASCVD risk along with CAC thresholds of >100 or >400. The RCT was assumed to be a 2 arm, 2-sided analysis, alpha error of 0.05, and have a statistical power of 80%. They were able to use these parameters along with the projected incidence of CVD events to extrapolate the sample size needed to adequately power each subpopulation's hypothetical RCT.8
The required sample sizes and the NNS together with published estimates of yearly cost per RCT participant allowed the authors to project a cost for each hypothetical RCT scenario by its specific entry criteria.9,10 To screen participants, the authors assumed a hypothetical cost of $600 per patient screened utilizing CAC (or $500 otherwise). For patients ultimately included in the RCT, they assumed a $6,000 annual expense per patient per year for the projected 5-year study. Sensitivity analyses also modeled an annual expense of $9,000 per participant.
Results
The NNS was shown to increase in each subpopulation along with increases in the target CAC score. The lowest NNS for each tier of CAC score were seen in the ASCVD ≥20% subpopulation. Similarly, the burden of observed CVD events was shown to rise in each subpopulation along with increases in observed CAC score.
The sample size required was shown to decrease along with ASCVD risk. Within each subpopulation, the additional entry criteria of CAC of >400 was associated with the lowest sample size. Overall, the combined criteria of ASCVD >10% and CAC of >400 was associated with the lowest sample size in both the RRR of 15% and RRR of 25% scenarios.
The authors' calculations revealed that projected cost did decrease along with increasing estimated ASCVD risk in both the RRR scenarios — e.g., assuming 15% RRR with novel therapy, projected costs were $623M with entry criteria of ASCVD ≥7.5% versus $402M with entry criteria of ASCVD ≥20%. Projected costs were markedly reduced with the addition of CAC of >400 as inclusion criteria — in the ASCVD ≥7.5% group, it alone reduced projected costs from $623M to $296M (assuming 15% RRR).The absolute lowest projected cost was found when researchers utilized the inclusion criteria of ASCVD ≥10% and CAC of >400 — assuming 15% RRR, projected costs were $283M; assuming 25% RRR, projected costs were $97M.
The authors investigated other potential RCT entry criteria outside of ASCVD risk and CAC (hsCRP levels, ankle-brachial index, etc.) as well as scenarios with alternative assumed expenses, and the above set of criteria remained superior.
Conclusions and Implications
While the addition of CAC of >400 modestly increased both screening costs and more than six-fold increased the NNS in the eventual lowest-cost ASCVD population ≥10% (NNS=6.1), that increased expense at study-outset would decrease overall expenditure dramatically and would result in the enrollment of a higher-risk study population. Thus, the sample size required would be much smaller than with other designs. Further, this study assumed that each patient would require a CAC scan de novo at the time of screening. As CAC scans become a part of more and more patients' charts, this will become the case less frequently.
One can see the financial impact the addition of CAC score thresholds as inclusion criteria can have in potential primary prevention cardiovascular RCTs. It can be reasonably inferred that CAC scores can increase efficiency elsewhere in the cardiovascular realm, allowing clinicians to select with even greater precision candidates for novel or costly therapies who may derive the most benefit. It is the responsibility of clinicians and health care systems to be judicious with the resources available to us, and selection of primary prevention patients with at least moderate CAC scores would seem to be helpful in this regard.
Figure 1
*The randomized controlled trial was assumed to be 2-sided, with an alpha error of 0.05 and statistical power of 80%, with a relative risk reduction with novel add-on therapy of 15% and using an estimated atherosclerotic cardiovascular disease risk threshold of $7.5%, which is the American College of Cardiology/American Heart Association guideline threshold for statin eligibility. †Assuming costs of $6,000/year per included participant and of $500 per screened nonparticipant ($600 for coronary artery calcium–based enrichment strategies).
References
- Center for Drug Evaluation and Research. Advancing Health Through Innovation 2017: New Drug Therapy Approvals (FDA website). 2018. Available at: https://www.fda.gov/files/about%20fda/published/2017-New-Drug-Therapy-Approvals-Report.pdf . Accessed 08/30/2021.
- Cainzos-Achirica M, Bittencourt MS, Osei AD, et al. Coronary artery calcium to improve the efficiency of randomized controlled trials in primary cardiovascular prevention. JACC Cardiovasc Imaging 2021;14:1005-16.
- Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871-81.
- The Multi-Ethnic Study of Atherosclerosis (MESA) (MESA website). 2021. Available at: https://www.mesa-nhlbi.org. Accessed 08/30/2021.
- Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289-97.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168-3209.
- Arnett DK., Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74:e177-e232.
- Sample Size Calculator (ClinCalc.com.). 2021. Available at: https://clincalc.com/stats/samplesize.aspx. Accessed 08/30/2021.
- Speich B, von Niederhäusern B, Schur N, et al. Systematic review on costs and resource use of randomized clinical trials shows a lack of transparent and comprehensive data. J Clin Epidemiol 2018;96:1-11.
- Moore TJ, Zhang H, Anderson G, Alexander GC. Estimated costs of pivotal trials for novel therapeutic agents approved by the US Food and Drug Administration, 2015-2016. JAMA Intern Med 2018;178:1451-57.
Clinical Topics: Arrhythmias and Clinical EP, Dyslipidemia, Prevention, Implantable Devices, SCD/Ventricular Arrhythmias, Nonstatins, Novel Agents, Statins
Keywords: Hydroxymethylglutaryl-CoA Reductase Inhibitors, Calcium, C-Reactive Protein, Cardiovascular Diseases, Prevalence, Ankle Brachial Index, Coronary Vessels, Health Expenditures, Pharmaceutical Preparations, United States Food and Drug Administration, Atherosclerosis, Risk Factors, Primary Prevention, Myocardial Infarction, Stroke, Heart Arrest
< Back to Listings