COVID-19 as a Possible Cause of Myocarditis and Pericarditis
Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) is an unmatched challenge for the healthcare community across the world. Respiratory involvement is the main clinical manifestation of COVID-19, ranging from mild flu-like illness to severe pneumonia, and potentially lethal acute respiratory distress syndrome.
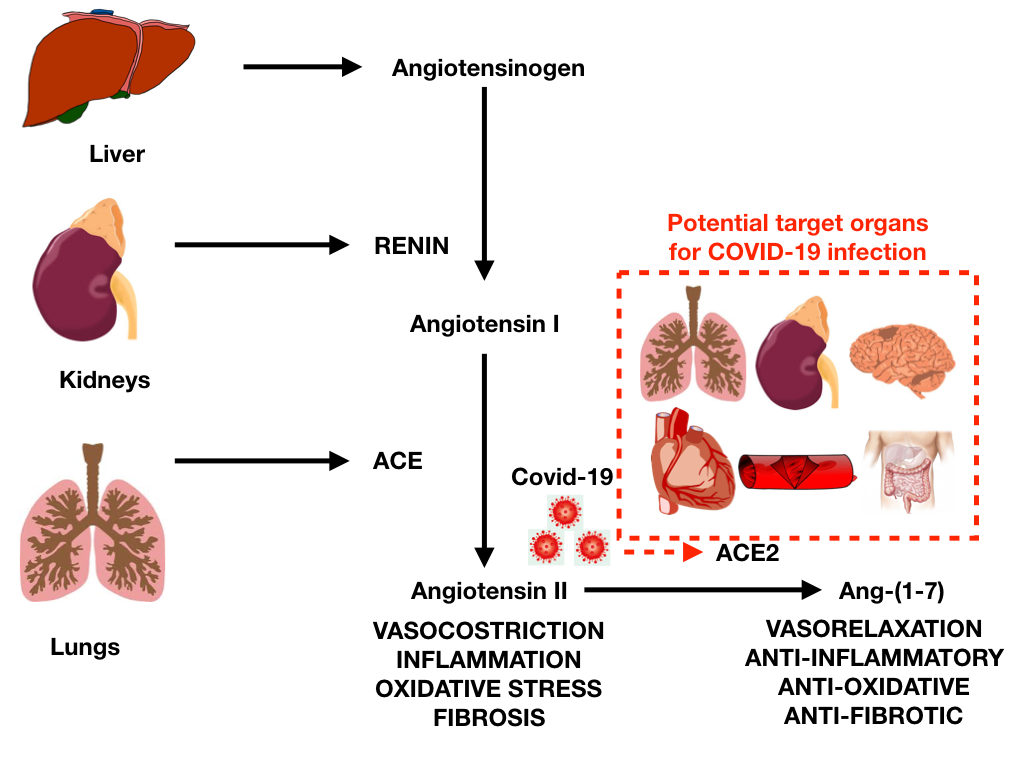
The initial mechanism for SARS-CoV-2 infection is viral binding to the membrane-bound form of angiotensin-converting enzyme 2 (ACE2) by a protein expressed in the viral coat, termed SPIKE (S protein) followed by its priming by the serine protease TMPRSS2 mediating virus uptake. ACE2 is a membrane-bound peptidase that is expressed in all tissues but is especially represented in lung, heart, vessels, kidney, brain, and gut (Figure 1).1
Figure 1
On this basis, all these organs are potential preferred targets for the virus, and this could explain several observations of lung, heart, vessel, brain and bowel complications, as well as symptoms and signs related to the involvement of these organs during COVID-19 infection.
Cardiovascular tissues or generally cells that express ACE2, including lung cells, are at risk for SARS-CoV-2 infection. Among cardiovascular complications, COVID-19 can be associated with myocarditis, pericardial effusion, and pericarditis (Table 1).1
Table 1: Reported Cardiovascular Complications in COVID-19
| Complication | Incidence | Notes |
| Acute cardiac injury* | 8-22% Up to 22% in ICU Up to 59% in those who died |
Most commonly reported cardiovascular complication |
| Pulmonary Thrombosis, Arterial and Venous Thromboembolism | 16-49% in ICU (case series) | Deep-vein thrombosis, PE, ischemic stroke, arterial thromboembolism |
| Heart Failure | Few data: 52% in those who died and 12% in those who recovered | Mechanisms not completely clear, probably related to myocardial lesion and septic shock |
| Acute coronary syndromes | Case reports: 44.4% with ST segment elevation on ECG had clinical diagnosis of MI, 50% underwent coronary angiography with detection of obstructive disease in 2/3. | High variability in presentation and prevalence of non-obstructive disease, poor prognosis |
| Arrhythmia | 16.7% overall; 44.4 in severe illness, 8.9% in mild cases | Both tachyarrhythmia and bradyarrhythmia can occur (limited data) |
| Myocarditis | Case reports | At present demonstration of SARS-Cov-2 in one report but in macrophages, not myocardial cells |
| Pericardial Diseases | Case reports | Pericardial effusions |
Myocardial cells are a potential target for SARS-CoV-2 and myocarditis has been reported in limited series from China, where 7% of deaths were attributed to myocardial damage with circulatory failure without a clear, definite diagnosis of myocarditis. Other reports have described fulminant myocarditis in the setting of high viral load with autopsy findings of inflammatory mononuclear infiltrate in myocardial tissue, but without evidence of direct COVID-19 infection in the myocardium.1-5
The diagnosis of myocarditis cannot be based only on troponin elevation, and additional evidence is often lacking. Most cases can be labeled as clinically suspected cases.
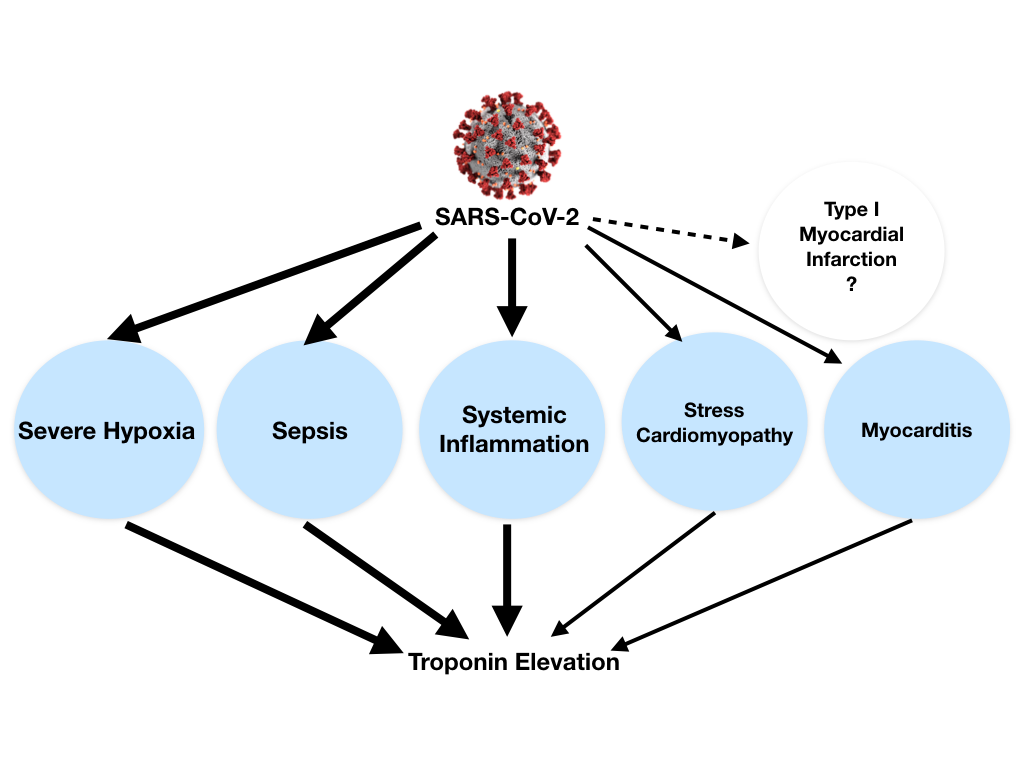
Troponin elevation in the setting of COVID-19 can be explained by different causes: (1) non-ischemic myocardial injury (more commonly) related to different possible mechanisms (e.g., severe hypoxia, sepsis, systemic inflammation, pulmonary thromboembolism, cytokine storm, stress cardiomyopathy) rather than a typical viral lymphocytic myocarditis, (2) ischemic myocardial injury with also different potential mechanisms (e.g., plaque rupture, coronary spasm, microthrombi, or direct endothelial or vascular injury). In both cases troponin elevation can be exacerbated by concomitant renal failure (Figure 2).1
Myocardial injury is prevalent among patients hospitalized with COVID-19. Moreover, troponin elevation among patients hospitalized with COVID-19 is associated with higher risk of mortality.2
Figure 2
In the setting of pericardial diseases, there are two possible different scenarios to consider: 1) the patient being treated for pericarditis who subsequently becomes infected with SARS-CoV-2, and 2) the patient with COVID-19 who develops pericarditis or pericardial effusion. In both conditions clinicians may be doubtful regarding the safety of non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, colchicine, and biological agents such as anti-IL1 agents (e.g., anakinra), that are mainstay of therapy for refractory cases.6
At present, limited data have been published on cases with COVID-19 who develop pericarditis and pericardial effusion. Most reported cases have been associated myocardial involvement with troponin elevation.
Although there is a warning on the use of NSAIDs in the setting of COVID-19 infection that require additional investigation, most treatments for pericarditis, including corticosteroids, colchicine, and anakinra do not appear contraindicated in the setting of COVID-19 infection (Table 2).6
Table 2: Current Treatments For Pericarditis and COVID-19 Infection
| Drug | Attack dose | Duration | LOE | Effect on COVID-19* |
| NSAIDs | Aspirin 750-1000mg x 3/day Ibuprofen 600-800mg x 3/day Indomethacin 25-50mg x 3/day |
1-2 weeks but until symptoms resolution and CRP normalization | A | Harmful (?) |
| Colchicine | 0.5mg x 2/day (0.5mg7day if <70Kg) |
3 months (acute) 6 months (recurrent) |
A | Potential therapy |
| Corticosteroid | 0.2 to 0.5mg/kg/day of prednisone | Up to 1 month | B | Therapy for advanced cases |
| Azathioprine | Up to 2 mg/kg | > 6 months | B | Unknown |
| NHIG | 400 to 500 mg/kg/day | 5 days (can be repeated after 1 month) | B | Potential therapy |
| Anakinra | 2mg/kg/day up to 100mg/day | 3-6 months then tapered | B | Potential therapy |
NSAID= non-steroidal anti-inflammatory drugs; NHIG= normal human immunoglobulins; LOE= Level of Evidence for pericarditis: A for multiple RCTs or metanalyses, B for a single RCT or observational studies, C for experts' consensus; CRP= C-reactive protein
*= current knowledge on the effect for COVID-19 infection, for corticosteroids and anakinra dosing for severe cased with COVID-19 is higher and intravenous: e.g., methylprednisolone 1mg/kg/day IvGTT for 7 days; anakinra IV infusion 4 times daily for 15 days. 400 mg/day in total, divided into 4 doses given every 6 hours.
Nevertheless, when corticosteroids and anakinra are used, a careful monitoring of possible superimposed bacterial infections is warranted. On this basis, pericarditis treatments should not be discontinued in patients on treatment when indicated to control the disease. As a matter of fact, these agents are currently being tested for efficacy in the setting of COVID-19 infection and could soon become part of the acknowledged treatment options.6
In conclusion, heart and vessels are potential targets for COVID-19, however at present, there are no findings which provide evidence of direct infection and replication of SARS-CoV-2 in heart cells. Additional pathologic studies and autopsy series will be very helpful to clarify the potentiality of SARS-CoV-2 to directly infect the myocardium/pericardium and cause myocarditis and pericarditis.
References
- Imazio M, Klingel K, Kindermann I, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart 2020;106:1127-31.
- Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76:533-46.
- Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J 2020;41:1861-62.
- Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz 2020;Mar 5:[Epub ahead of print].
- Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22:911-15.
- Imazio M, Brucato A, Lazaros G, et al. Anti-inflammatory therapies for pericardial diseases in the COVID-19 pandemic: safety and potentiality. J Cardiovasc Med 2020;21:625-29.
Clinical Topics: COVID-19 Hub, Heart Failure and Cardiomyopathies, Pericardial Disease, Vascular Medicine
Keywords: COVID-19, severe acute respiratory syndrome coronavirus 2, Myocarditis, Pericarditis, Peptidyl-Dipeptidase A, Cytokines, Virus Attachment, Serine Proteases, Troponin, Pericardial Effusion, Takotsubo Cardiomyopathy, Vascular System Injuries, Viral Load, Respiratory Distress Syndrome, Myocardium, Pulmonary Embolism, Sepsis, Renal Insufficiency, Kidney, Delivery of Health Care
< Back to Listings