Perspective | Recall of Valsartan-Containing Products: Clarion Call to Prepare Systematic Response
The recent recall of several valsartan-containing products not only raises numerous issues and concerns regarding the safety and purity of this widely used antihypertensive medication, it also highlights areas for improvement when it comes to future identification of contaminated products and responses to recall notices issued by the U.S. Food and Drug Administration (FDA).
The recall was initially issued when the FDA found that multiple valsartan-containing products contained a potential carcinogen. I first learned of the recall via a newsfeed from which I routinely receive notifications about medical, pharmaceutical and health issues. After going to the FDA website, I then notified my cardiology colleagues about the recall and the likelihood we would receive a torrent of phone calls from patients. I, and likely the vast majority of physicians, do not routinely check the FDA website for notifications of drug recalls and I would have missed this important alert if I had not received the other notification.
To make matters more complicated, the FDA named a number of generic pharmaceutical companies' products as being contaminated within its initial recall notice. Unfortunately, as it turned out, the list was incomplete, and products thought to be not contaminated were later identified as containing the contaminant.
In addition, initially, products containing valsartan-amlodipine (+/- hydrochlorothiazide) were said to be uniformly not included in the recall; this too turned out to be in error. Thus, notification of patients that their valsartan-amlodipine tablet (a very common and effective treatment regimen) was not included in the recall had to be rescinded when additional information was revealed by the FDA.
The shortcomings of this initial notification process point to two needs: 1) an effective means of distributing such information to all physicians who may prescribe these drugs; 2) notification that is complete and accurate, so action can be taken with all due speed to remove the drug from the treatment regimens of our patients and replace it with effective alternatives.
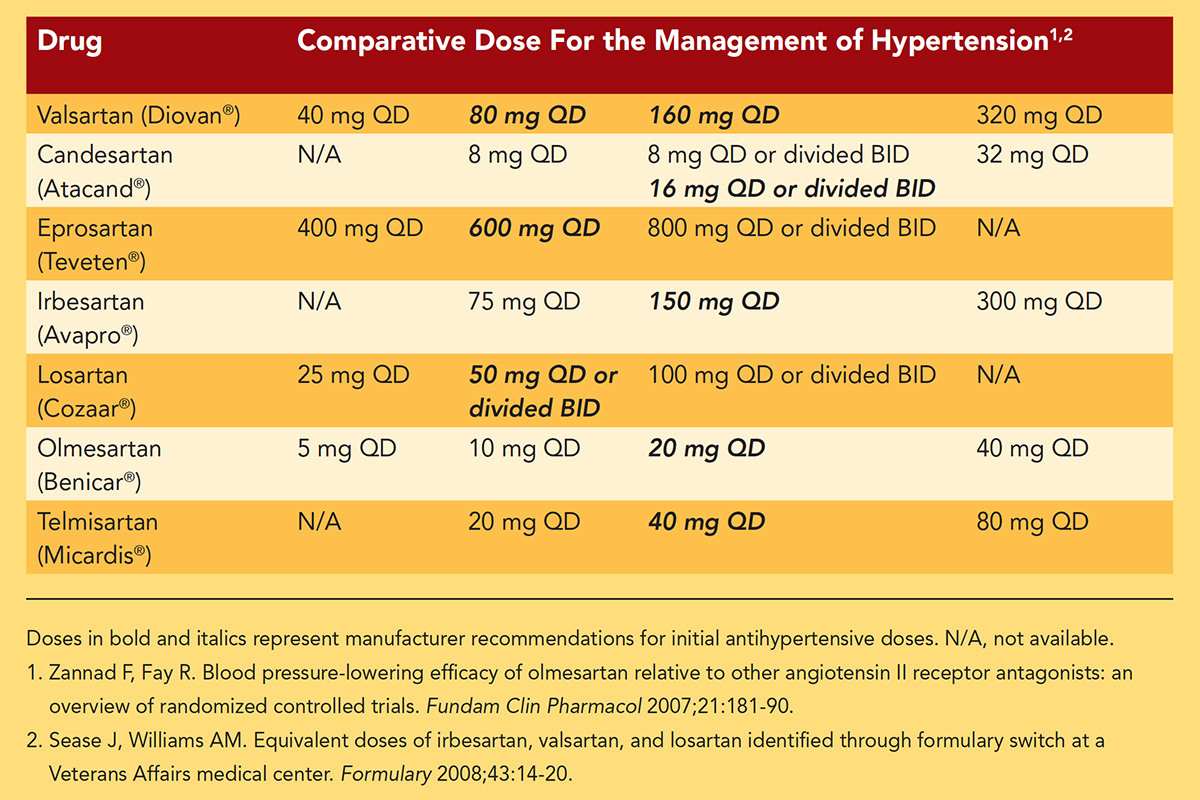
At my institution, the second phase of our response to the safety notice following initial contact with patients was to propose alternative medications to replace valsartan in the treatment of those taking this drug. Fortunately, valsartan, an angiotensin receptor blocker (ARB), is a member of a family of drugs with many alternate choices. Among the currently available alternatives (e.g., losartan, irbesartan, olmesartan, candesartan, telmisartan, to name a few) are many generic ARB products (and one non-generic product, azilsartan).
Key hospital committees, including pharmacy and safety committees, sent notifications to the medical staff about the valsartan recall and provided a guide for drug equivalencies for transitioning from valsartan to an alternative product.
It is important to note that these drug dosage potency equivalencies are based on populations of patients' responses and that individual responses to the alternative product may be greater or less than the response to valsartan based on individual rates of drug bioavailability, metabolism and excretion.
Thus, determining the individual patient's response to the newly-initiated drug by patient self-monitoring or a blood pressure measurement by a health care provider was an important part of the transition process.
Importantly, we also asked each clinician if they had a preferred replacement product and a dose-for-dose suggestion for that product. By identifying a general transition plan for most (if not all) patients who needed a new prescription sent to their pharmacy, our nursing staff was able to rapidly and effectively make these changes without asking the staff for patient-by-patient recommendations. The establishment of such a practice was essential to our effective response.
The next challenge was to identify and notify our patients of the need to identify the source of their product, whether it was part of the recall and what product to take in its place. Patients who had purchased their medication at our outpatient pharmacy and were part of our Patient Gateway Communication Portal received electronic notices. Those not participating in the portal were mailed letters.
In both cases, patients were asked to contact their health care provider, but not to abruptly discontinue their medication use until a replacement product was in hand. The ease and speed of communication via the Patient Gateway portal obviously allowed for faster, timelier and personalized communications, compared to other modes of communications – which in many cases took weeks.
Given the challenges with this latest recall, how do we go forward in an era of polypharmacy for the treatment of increasingly common cardiac disorders (hypertension, hyperlipidemia, atrial fibrillation, coronary artery disease, to name just a few)? Several questions come to mind.
First, when a contaminated product is identified, how can the FDA most effectively notify the health care community? These notifications need to be as complete as possible at the outset of the process; revisions to the products deemed safe or not undermines the effort to notify patients in an effective fashion.
FDA Recalls Additional Hypertension Medication Due to Contamination With Probable Carcinogen

On Oct. 30, the U.S. Food and Drug Administration (FDA) announced a voluntary recall by ScieGen Pharmaceuticals, Inc. for several lots of irbesartan in USP 75 mg, 150 mg and 300 mg tablets. These products are being recalled due to the presence of N-nitrosodiethylamine (NDEA), an impurity classified as a probable human carcinogen by the International Agency for Research on Cancer.
The irbesartan tablets (USP 75 mg, 150 mg and 300 mg) subject to recall are labeled as Westminster Pharmaceuticals and Golden State Medical Supply, Inc. and are packed in 30-count and 90-count bottles. Review a list of the recalled lot numbers here. To date, ScieGen Pharmaceuticals has not received any reports of adverse events related to this product.
The ACC is closely monitoring the recalls of both irbesartan and valsartan and will continue to provide members with all available information as the situation develops.
However, delay in notification cannot be accepted just for the sake of testing every single product in the marketplace. Unfortunately, an initial indictment of a product, later to be found safe, is probably less onerous than an initial clearance of that product only later to be found to be contaminated and thus recalled.
Second, the availability of alternative choices to replace a recalled product is essential. Fortunately, in this case this was not a problem. But, how would we have handled the situation if alternatives were not as easily identified – resulting in millions of persons having to be switched to alternative products of a different mechanism of action, necessitating many iterations of testing for an effective reduction in blood pressure. The availability of alternative drugs having the same mechanism of action made the current response much easier than it might have been.
Similarly, multiple producers of the product are essential so they might "step in" and fill any voids in the supply chain. Non-generic valsartan, marketed by Novartis as Diovan, the original product, was always an alternative for valsartan-responsive patients and many chose to turn to Diovan; however, the cost of Diovan, as reported to me by a number of patients, substantially exceeds that of generic valsartan and other generic products.
Finally, we need to identify a more (or most) effective strategy for identifying patients taking a certain product. The news media, social media, pharmacy notifications, pharmacy benefit plans notifications, hospital communication portals, etc., all contributed to an effective, if not time consuming, process of identifying patients who may have needed to transition to alternative products.
However, our dependence on the electronic health record (EHR) also should have provided an opportunity for the near-immediate identification of the patients in need of notification. It is alleged that we should be able to "search" the medication lists of all of our patients and thereby identify each and every patient for whom a drug (in this case, valsartan) had been prescribed.
To avoid future and indeed potentially more complicated circumstances, every health care institution should put in place a system that with just a few keystrokes allows for their EHR to be scanned, those at risk identified and immediate electronic and/or physical notifications sent to those patients. It is not a question of if we will need to do this again, but when. This experience should serve as a clarion call of the need to be prepared for future events, which are likely, unfortunately, to be more and more frequent.

This perspective was authored by Randall M. Zusman, MD. He is the director of the division of hypertension at the Massachusetts General Hospital Heart Center and associate professor of medicine at Harvard Medical School.
Clinical Topics: Arrhythmias and Clinical EP, Dyslipidemia, Prevention, Atherosclerotic Disease (CAD/PAD), Atrial Fibrillation/Supraventricular Arrhythmias, Novel Agents, Hypertension
Keywords: ACC Publications, Cardiology Magazine, Amlodipine, Angiotensin Receptor Antagonists, Antihypertensive Agents, Atrial Fibrillation, Benzimidazoles, Benzoates, Biological Availability, Biphenyl Compounds, Blood Pressure, Carcinogens, Coronary Artery Disease, Diethylnitrosamine, Drug Recalls, Electronic Health Records, Health Personnel, Hyperlipidemias, Hypertension, Imidazoles, International Agencies, Losartan, Medical Staff, Neoplasms, Nursing Staff, Outpatients, Oxadiazoles, Polypharmacy, Social Media, Tablets, Tetrazoles, United States Food and Drug Administration
< Back to Listings