Cover Story | Intravascular Imaging: Understanding the Role of IVUS and OCT

Angiography provides a detailed, 2D image for the assessment of intravascular lesions. Despite its routine use to perform complex coronary and peripheral interventions, angiography has several limitations when compared with higher resolution intravascular imaging techniques. Complex lesion anatomy and significant inter-operator variability in angiogram interpretation limit the ability to accurately predict lesion architecture and hemodynamic significance.
Intravascular images eliminate some of the technical difficulties inherent to angiography, such as contrast streaming artifact, vessel overlap and suboptimal ostial views. Several modalities are available to further classify lesions, including fractional flow reserve (FFR), intravascular ultrasound (IVUS) and optical coherence tomography (OCT).
As opposed to the luminogram obtained by angiography, IVUS and OCT produce cross-sectional images capable of accurately determining vessel size and plaque morphology. Angiographically “normal” vessels or vessel segments may be found to have diffuse disease that occupies a significant proportion of the true lumen.
IVUS and OCT images have far greater spatial resolution compared with angiography, resulting in more accurate lesion characterization. Details such as lesion length, eccentricity, calcification, thrombus, necrotic cores, dissections and stent apposition/expansion are easily visualized. A detailed description of IVUS and OCT including advantages and disadvantages of each is discussed here. Figures 1 and 2 are representative images obtained by IVUS and OCT of a mixed plaque and malapposed stent, respectively.
IVUS and OCT: Advantages and Disadvantages
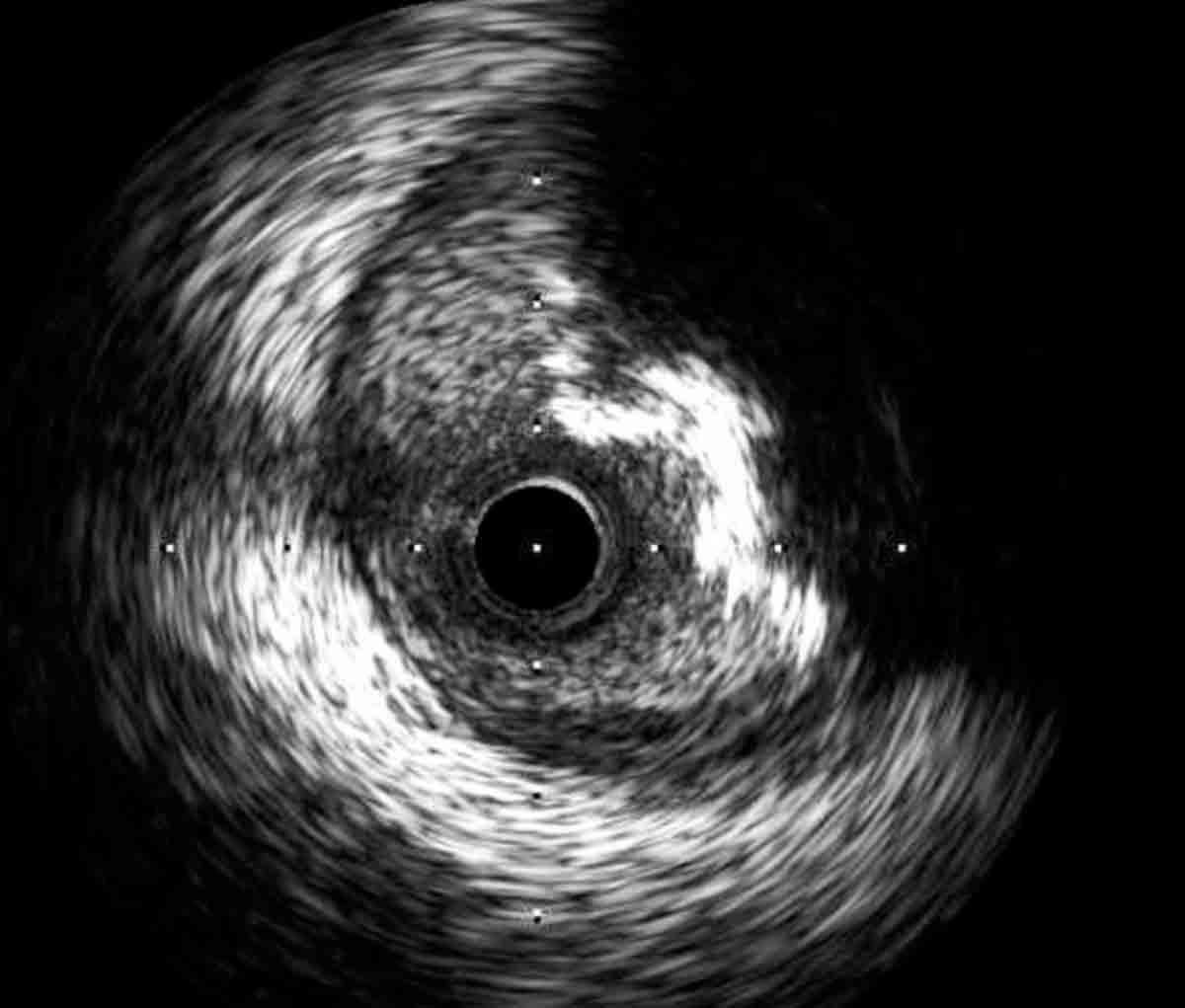 Figure 1: IVUS image of a mixed plaque in the proximal left anterior descending artery.
Figure 1: IVUS image of a mixed plaque in the proximal left anterior descending artery.
IVUS images are generated using high-frequency sound waves from a piezoelectric crystal (10 to 40 MHz) to penetrate tissue, reflect off vascular structures and return to the transducer to produce multidimensional images of the vessel. The device is mounted on a single catheter. Tissue penetration is approximately 4 to 8 mm. Standard IVUS catheters are advanced over a 0.014-inch coronary guidewire through a 5 French (F) or larger guide. Standard anticoagulation with heparin or bivalirudin is required. The catheters are 3.2 to 3.5 F with a tapered tip.1
Images are obtained as the catheter is retracted from the distal to the proximal vessel. The motorized pullback retracts the catheter at approximately 0.5 mm/second; manual pullback can be performed too. The piezoelectric crystal may be attached to a rotating internal mechanism or controlled electronically in a solid-state system.
The axial resolution of IVUS images is currently 100 to 150 microns. A trilaminar image is created with the normal vessel having an innermost intima, middle media and outermost adventitia. Measurements of the lumen can be performed manually or with automated software.1,2
Typical indications for performing IVUS include determining stenosis severity, evaluating extent of vessel calcification, sizing of the vessel, discerning the mechanism of stent failure and characterizing lesion composition. Several artifacts are specific to IVUS and may interfere with image interpretation.
The most commonly encountered artifacts are guidewire artifact, acoustic shadowing from calcium, ring down artifact, blood speckle artifact from increased transducer frequency or decreased blood velocity, and nonuniform rotational distortion (NURD) seen in rotational systems due to binding of the drive cable which rotates the transducer.3
OCT images are obtained using near infrared light (approximately 1300 nm) emitted from a single fiber optic wire that reflects off vascular structures and returns to a detector. Post processing creates a high-resolution image. The principal advantage of OCT over angiography and IVUS is higher axial and lateral resolution. Axial resolution is typically less than 10 to 20 microns.
Tissue penetration is approximately 1 to 3 mm, due to the wavelength of light utilized. Deeper penetration can be achieved with longer wavelengths. However, the optimal wavelength is also limited by tissue absorption and light mechanics. The catheters are advanced over a 0.014-inch coronary guidewire through at least a 5 F guide, although 6 F is preferable.
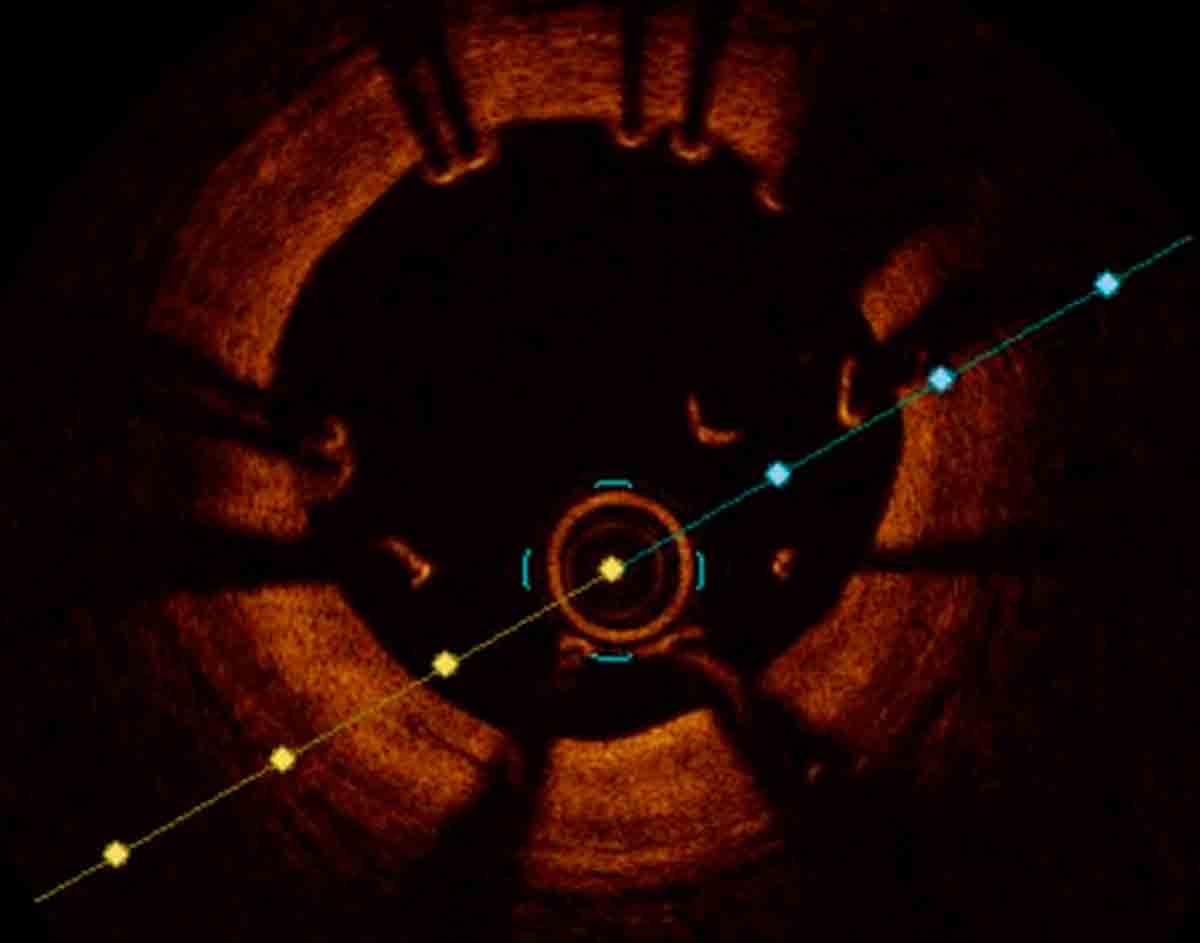 Figure 2: OCT image of a malapposed stent in the mid left anterior descending. Additional post dilation correctly apposed the stent to the vessel wall.
Figure 2: OCT image of a malapposed stent in the mid left anterior descending. Additional post dilation correctly apposed the stent to the vessel wall.
OCT images are obtained in a bloodless environment as the catheter is withdrawn from the distal to proximal vessel. The blood in the vessel is removed by a contrast injection during image acquisition, typically 10 to 15 cc. First-generation devices required proximal vessel balloon occlusion followed by crystalloid flush to clear the artery. This resulted in prolonged ischemic time and is no longer required. The pullback occurs more rapidly than IVUS, at approximately 25 mm/second. The optical fiber rotates at 100 revolutions/second. Anticoagulation with heparin or bivalirudin is required. Standard OCT catheters are approximately 3.2 F with a tapered tip.1,4
Artifacts typically encountered during OCT imaging include guidewire artifact, residual intraluminal blood resulting in light attenuation, “sunflower effect” due to distortion of stent reflections from eccentric wire position, stitch artifact from rapid catheter or wire movements, and artifacts from air bubbles. Proper guidewire position and contrast flushing will help minimize these artifacts.4,5
The overall safety of each device has been extensively evaluated. One of the largest of these studies included 5,148 IVUS and 1,142 OCT pullbacks with a complication rate of 0.2 percent. The most frequent coronary complication is vasospasm which generally resolves with time and intracoronary vasodilators. More serious or life-threatening complications such as coronary dissection or perforation occur at a much lower rate.6
Thrombus formation on the catheters or stent dislodgement with pullback have been reported but are exceedingly rare.7 The amount of energy delivered with each modality is relatively small and has not been proven to cause any significant intimal damage. There may be additional renal risk associated with the contrast injection for OCT, however this does not appear to be significant. The additional contrast used to flush the catheter contributes minimally to the overall contrast use.4,8
Both IVUS and OCT generate an anatomical map of the vessel. The minimal and maximal lumen diameters and cross-sectional areas can be determined. These measurements are critical to determining the significance of a lesion and selecting an appropriate stent size.3 The best data correlating minimal luminal area (MLA) with FFR is in left main stenosis using IVUS. In the absence of significant aorto-ostial disease, OCT also can be used to size the left main. With a significant ostial lesion, full guide engagement to clear the artery of blood may not be possible, producing a significant amount of artifact.
A left main MLA of <5.9 mm2 generally correlates with an FFR of <0.75.9 Extrapolating cutoffs across demographics may lead to a decreased sensitivity for this value. Kang and colleagues showed in a Korean population that 4.8 mm2 was the most appropriate MLA cutpoint.10 In non-left main lesions, caution must be used when attempting to correlate MLA with functional outcomes.11
The reasons for this were described by Pijls and colleagues and includes lesion location in the coronary tree, entrance and exit angles, shear forces, reference vessel dimensions and the amount of viable myocardium supplied by the vessel.12
Stent diameter selection is typically driven by the smallest reference vessel diameter that corresponds to the distal vessel. Fortunately, modern second-generation drug-eluting stents (DES) can be upsized significantly from the nominal diameter. This allows for post dilation to the correct size.
Bioresorbable stents created to date do not have this capability, making correct sizing even more crucial.13 Finally, intravascular imaging helps identify appropriate proximal and distal landing zones for intracoronary stents, leading to more appropriate stent lengths.
A wide variety of intraluminal abnormalities can be identified with both IVUS and OCT. Accurate plaque characterization is crucial to planning a successful intervention. With the exception of structures located deep in the vessel wall, most lesions are assessed in finer detail with OCT.1,4 Specific abnormalities of interest to the interventionalist include thrombus, neointimal hyperplasia, lipid plaque, fibrous cap and calcium. More specific tissue characterization with OCT has the ability to distinguish white from red thrombus.14
The higher resolution of OCT allows for precise measurements of the fibrous cap. Recent studies have shown a thicker fibrous cap in persons who experience acute myocardial infarction (MI) during exertion compared with those at rest.15 There is also a significant increase in fibrous cap thickness with the use of statins.16 Studies examining the ability of OCT to identify vulnerable plaques with a high degree of inflammation and risk of rupture are ongoing.
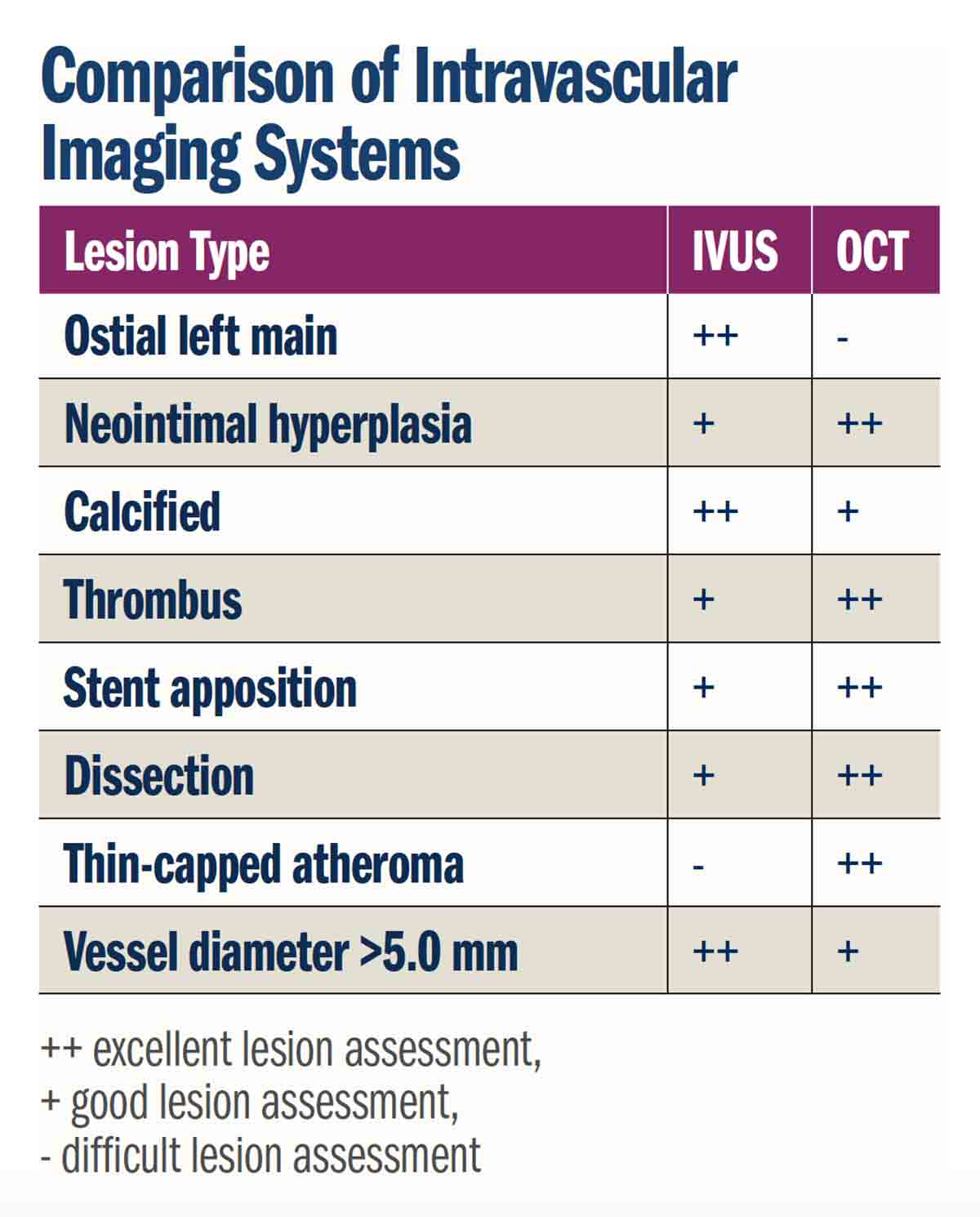
Determining the degree of lesion calcification aids in planning a revascularization technique. Heavy calcification is associated with stent under-expansion and adverse events.17 As opposed to angiography, IVUS and OCT can quantify the circumference of the artery involved with calcium. Eccentric calcium and localized nodules can also be visualized.4,18
OCT, but not IVUS, can determine the thickness of a calcified plaque. Plaque modification with rotational or orbital atherectomy decreases the risk of stent under-expansion. There is no consensus as to the arc or degree of calcification that should undergo plaque modification prior to stenting. Most operators err on the side of upfront atherectomy for heavily calcified lesions, due to the risk associated with stent under-expansion.13
In addition to evaluating de novo stenoses, intravascular imaging is crucial to determining the mechanism of acute stent thrombosis and in-stent restenosis (ISR). Acute stent thrombosis is a rare but potentially fatal event. There are multiple potential mechanical mechanisms including stent fracture, edge dissection, stent under-expansion and stent malapposition. These defects are easily detected by intravascular imaging.19,20
ISR rates significantly decreased with the advent of DES, however this continues to be a frequently encountered clinical problem. Kang and colleagues analyzed 79 IVUS-identified cases of ISR. Luminal areas were less than 4 mm2 and the lesions were defined as focal (≤10 mm), multifocal (≥1 focal lesion) or diffuse (>10 mm in length). Overall, 42 percent had stent under-expansion and 93 percent had significant intimal hyperplasia.21
Choosing a modality of intravascular imaging is typically dependent on the clinical scenario and operator preference. IVUS is primarily limited by its lower image resolution, however it provides adequate visualization to determine vessel size, extent of calcification and identify thrombus. The higher image resolution of OCT allows for a more detailed evaluation to determine the presence of stent malapposition and intimal tears.
Penetration into the vessel wall is limited with OCT due to its light-based technology. This makes the evaluation of more external vessel structure more challenging. Ostial lesions are more easily evaluated with IVUS as noted previously. Image acquisition is similar with both techniques, except for the need to displace the blood pool prior to OCT imaging, which is required because light is rapidly attenuated by blood.1
Clinical Evidence for PCI Guided by IVUS, OCT, Angiography
Numerous studies have investigated IVUS-guided vs. angiography-guided PCI. These have shown significant improvements in cardiac outcomes with intravascular imaging. Proposed mechanisms include critical evaluation of important stent characteristics, including stent apposition, stent expansion, minimal cross-sectional area and luminal gain. Two meta-analyses in the bare-metal stent era showed decreased rates of restenosis and repeat revascularization, but not MI or death in patients who underwent IVUS-guided PCI.22,23
Multiple trials have been performed with DES. The largest meta-analysis of the DES trials included 29,068 patients and showed a significant reduction in major adverse cardiovascular events (MACE) (hazard ratio [HR], 0.77); MI (HR, 0.64); death (HR, 0.62); target lesion revascularization (HR, 0.81); and target vessel revascularization (HR, 0.86) (p<0.001 for all) compared with angiography alone.24
Seven randomized, controlled studies were evaluated in a meta-analysis by Elgendy et al., which showed decreased rates of stent thrombosis, ischemia-driven target vessel revascularization and death in patients who underwent IVUS vs. angiography-guided PCI. This study included a total of 3,192 patients.25 Additional, nonrandomized studies including significantly more patients have shown similar results.26,27
Patients with more complex coronary disease likely benefit from a revascularization approach that includes intravascular imaging. In the recent left main trials EXCEL and NOBLE, IVUS was used in greater than 70 percent of cases.28,29 In a propensity matched analysis, de la Torre Hernandez and colleagues demonstrated the unreliable nature of angiography for assessment of left main stenoses. Using an MLA cutoff of 6 mm2 cardiac death and event-free survival were not significantly different between the deferred and revascularized groups.30
Chronic total occlusion (CTO) lesions present complex challenges for the interventionalist. Many of the CTO lesions can be preempted with intravascular imaging. Kim and colleagues showed a lower MACE rate of 2.6 percent vs. 7.1 percent (p=0.035) using IVUS in 402 patients undergoing percutaneous CTO interventions randomized to IVUS or angiographic guidance.31
Intracoronary Imaging: Insights From an Expert
Eric Osborn, MD, PhD, FACC, is director of the Interventional Cardiology Fellowship Program at Beth Israel Deaconess Medical Center in Boston, MA, and instructor in medicine at Harvard Medical School. He is the principal investigator of the NIH/NHLBI grant Intravascular Molecular-Structural Imaging of Coronary Stent Pathobiology.
Osborn talked with Mark Tuttle, MD, FIT section editor for Cardiology: Interventions, about his research in intracoronary imaging, its clinical role and what may lie ahead for an expanding role. READ MORE.
Certainly, interventions on bifurcation lesions can be optimized with IVUS or OCT by allowing for optimal stent sizing, assessment of ostial side branch disease and lesion length. Finally, as mentioned previously, ISR continues to be a clinical problem. The greater resolution of OCT allows for more precise identification of neointimal hyperplasia and undersized or under-expanded stents. This information is invaluable for developing a revascularization strategy.2
There is a growing body of literature comparing IVUS and OCT. The OPINION and the ILUMIEN II and III trials have shown that OCT is at least equivalent to IVUS for most indications. OPINION demonstrated a trend toward larger postprocedure in-stent angiographic minimum lumen diameter (2.63 vs. 2.56 mm; p=0.058) with IVUS compared with OCT.
ILUMIEN III found that the final minimal stent area following OCT-guided PCI was noninferior to that of IVUS-guided PCI (5.79 vs. 5.89 mm2; p=0.12).32-34 The several-fold greater sensitivity of OCT for acute stent malapposition has not resulted in more favorable long-term outcomes.
Studies have consistently shown a marginally higher dose of contrast with OCT compared with IVUS. Due to the higher resolution of OCT, minor complications of PCI such as small edge dissections, minimal thrombus adherent to stent struts and areas of slight stent malposition are often identified. More research is necessary to determine the significance of small defects.35
The 2011 guideline for PCI from the ACC, American Heart Association and Society for Cardiovascular Angiography and Interventions recommends the use of IVUS for evaluating indeterminate left main lesions and indeterminate non-left main lesions (50 to 70 percent stenosis).36
Additional recommended uses include determining the etiology of ISR and stent thrombosis.37 OCT has yet to enter published guidelines, however there is mounting evidence for its use and guideline recommendations are likely forthcoming.
This article was authored by Stephen Gannon, MD, an interventional cardiology fellow at the Brigham and Women’s Hospital in Boston, MA.
References
- Koganti S, Kotecha T, Rakhit RD. Interv Cardiol 2016;11:11-16.
- Rathod KS, Hamshere SM, Jones DA, Mathur A. Interv Cardiol 2015;10:8-15.
- Vasquez A, Mistry N, Singh J. Interv Cardiol 2014;9:156-63.
- Bezerra HG, Costa MA, Guagliumi G, et al. JACC Cardiovasc Interv 2009;2:1035-46.
- Elahi S, Mancuso JJ,Milner TE, Feldman MD. JACC Cardiovasc Imaging 2011;4:1220-1.
- van der Sijde JN, Karanasos A, van Ditzhuijzen NS, et al. Eur Heart J Cardiovascular Imaging 2017;18:467-74.
- Yamaguchi H, Takaoka J, Miyamura A, et al. JACC Cardiovasc Interv 2012;5:690-1.
- Barlis P, Gonzalo N, Di Mario C, et al. EuroIntervention 2009;5:90-5.
- Jasti V, Ivan E, Yalamanchili V, et al. Circulation 2004;110:2831-6.
- Kang SJ, Lee JY, Ahn JM, et al. JACC Cardiovasc Interv 2011;4:1168-74.
- Abizaid A, Mintz GS, Pichard AD, et al. Am J Cardiol 1998;82:423-8.
- Pijls NH, Sels JW. J Am Coll Cardiol 2012;59:1045-57.
- Bezerra HG. Intravascular OCT in PCI. June 13, 2016. Available here. Accessed August 6, 2018.
- Kume T, Akasaka T, Kawamoto T, et al. Am J Cardiol 2006;97:1713-7.
- Tanaka A, Imanishi T. Kitabata H, et al. Am J Cardiol 2008;102:975-9.
- Takarada S, Imanishi T, Kubo T, et al. Atherosclerosis 2009;202:491-7.
- Mintz GS. JACC Cardiovasc Imaging 2015;8:461-71.
- Bezerra HG, Attizzani GF, Sirbu V, et al. JACC Cardiovasc Interv 2013;6:228-36.
- Alfonso F, Suarez A, Angiolillo DJ, et al. Heart 2004;90:1455-9.
- Adriaenssens T, Joner M, Godschalk TC, et al. Circulation 2017;136:1007-21.
- Kang SJ, Mintz GS, Park DW, et al. Circ Cardiovasc Interv 2011;4:9-14.
- Casella G, Klauss V, Ottani F, et al. Catheter Cardiovasc Interv 2003;59:314-21.
- Parise H, Maehara A, Stone GW, et al. Am J Cardiol 2011;107:374-82.
- Zhang YJ, Pang S, Chen XY, et al. BMC Cardiovasc Disord 2015;15:153.
- Elgendy IY, Mahmoud AN, Elgendy AY, Bavry AA. Circulation Cardiovasc Interv 2016;9:e003700.
- Ahn JM, Kang SJ, Yoon SH, et al. Am J Cardiol 2014;113:1338-47.
- Klersy C, Ferlini M, Raisaro A, et al. Int J Cardiol 2013;170:54-63.
- Stone GW, Sabik JF, Serruys PW, et al. N Engl J Med 2016;375:2223-35.
- Makikallio T, Holm NR, Lindsay M, et al. Lancet 2016;388:2743-52.
- de la Torre Hernandez JM, Hernandez Hernandez F, Alfonso F, et al. J Am Coll Cardiol 2011;58:351-8.
- Kim BK, Shin DH, Hong MK, et al. Circ Cardiovasc Interv 2015;8:e002592.
- Kubo T, Shinke T, Okamura T, et al. J Cardiol 2016;68:455-60.
- Maehara A, Ben-Yehuda O, Ali Z, et al. JACC Cardiovasc Interv 2015;8:1704-14.
- Ali ZA, Maehara A, Genereux P, et al. Lancet 2016;388:2618-28.
- Meneveau N, Ecarnot F, Souteyrand G, et al. Am Heart J 2014;168:175-181.e1-2.
- Levine GN, Bates ER, Blankenship JC, et al. J Am Coll Cardiol 2011;58:e44-e122.
- Lotfi A, Jeremias A, Fearon WF, et al. Catheter Cardiovasc Interv 2013;83:509-18.
Clinical Topics: Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Interventions and Imaging, Angiography, Nuclear Imaging
Keywords: ACC Publications, Cardiology Interventions, Tomography, Optical Coherence, Artifacts, Coronary Angiography, Angiography, Hemodynamics
< Back to Listings




