Interactions of Colchicine Within Cardiovascular Medicine
A 50-year-old man with a history of hypertension, hyperlipidemia, and an ischemic cardiomyopathy presents to the emergency department complaining of one-day history of excruciating chest pain, sudden in onset described as sharp and stabbing in nature with exacerbation on deep inspiration located over the center of the chest with suggestion of radiation to the left shoulder blade. The pain worsens when he is in the supine position and improves when leaning forward. The patient recalls having a runny nose and some congestion the prior week. Home medications include aspirin, atorvastatin, losartan, carvedilol, and ranolazine. An ECG is obtained revealing:
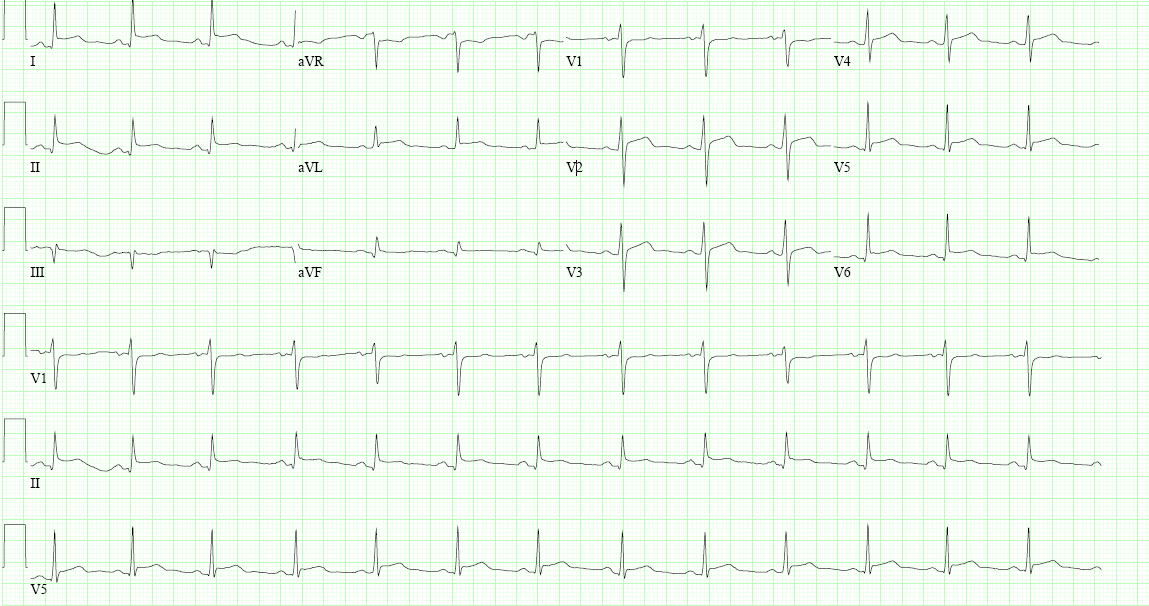
Serial ECGs 30 minutes apart remain unchanged. The initial work up revealed normal renal and liver function, normal cardiac troponin, and elevation in inflammatory markers (C-reactive protein and erythrocyte sedimentation rate). An echocardiogram was also obtained which is shown below:
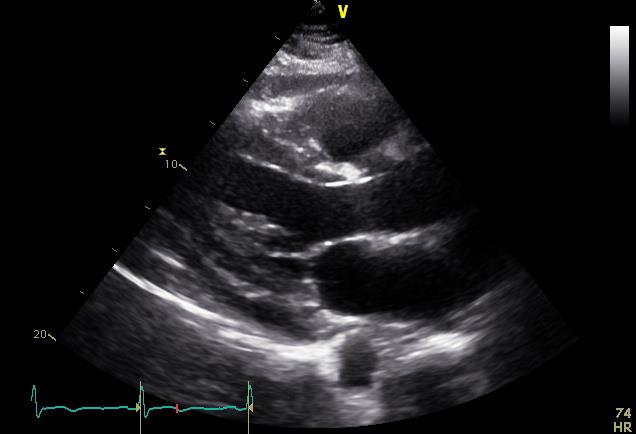
The patient is diagnosed with acute pericarditis; thus, the physician recommends a 2-week course of ibuprofen with 3 months of adjunctive colchicine. The patient is concerned about adding colchicine to his regimen; he wonders if there is any potential interaction of colchicine with his current medications.
Which of the following medication increases the risk of colchicine toxicity via drug-drug interaction?
Show Answer
The correct answer is: D. All of the above.
Colchicine is one the oldest drugs known to man and still plays an important role in our current therapeutic pharmacopeia. After decades of use chiefly for gout attacks and familial Mediterranean fever (FMF), the utility of colchicine is now established as a frontline agent for the management of acute and chronic pericarditis including post-pericardiotomy syndrome.1,2 A growing body of evidence suggests a potential role in subjects with stable coronary artery disease and atrial fibrillation.3,4 Colchicine has a low therapeutic index with well-known side effects ranging from gastrointestinal symptoms, alopecia, myopathy and neuropathy to myelosuppression, multi-organ failure, and death.3 More importantly, there exist reports of patients dying from colchicine toxicity even when the drugs is prescribed at therapeutic doses, possibly due to interaction with co-administered drugs or in the setting of reduced drug clearance.5,6
The safety profile of colchicine is well-established when used for short term (<5 weeks) for the management of gout flares and FMF;5 however, less is known when the drug is used for rather prolonged periods of time and adjunctive to other cardiovascular drugs. Reports of side effects and toxicity of colchicine from cardiovascular trials are inconsistent; the main side effects are gastrointestinal symptoms in up to a fifth of subjects with no mortality reported.1 Nevertheless, no clinical studies to date have addressed the potential interaction of colchicine with commonly prescribed medications in the cardiovascular pharmacopeia. Therefore, until more data about colchicine safety become available, cardiologists could use pharmacodynamics data to minimize drug-drug interactions between colchicine and other cardiovascular medications. In addition, the existent data on interactions of digoxin, another P-glycoprotein (P-gp) substrate, in cardiovascular medicine could help predict potential interaction of colchicine with commonly used cardiovascular drugs via P-gp.7
Colchicine primarily undergoes liver metabolism via cytochrome P450 3A4 (CYP3A4) followed by hepatobiliary elimination. In addition, about 10-20% of the drug is renally eliminated using the P-gp ATP efflux transporter.8 In addition to being responsible for the renal excretion, the P-gp system regulates the concentration of colchicine in plasma by modulating the gastrointestinal absorption of the drug.3 Many cardiovascular drugs interact with the CYP3A4 and/or the P-gp efflux system; therefore careful revision of medications should occur before prescribing colchicine.7 This is important as drug-drug interactions can lead to either early colchicine discontinuation therapy or toxicity.
Our index patient is taking aspirin, atorvastatin, losartan, carvedilol, and ranolazine. In regards to antiplatelet therapy, there is no evidence to support that aspirin, clopidrogel, or prasugrel could lead to toxicity via drug-drug interaction with colchicine. Aspirin induces the P-gp system, clopidrogel is only a substrate, while prasugrel does not interact with the efflux system.7 Nonetheless, ticagrelor moderately inhibits the P-gp system in a dose dependent manner with the potential to increase colchicine concentrations.7
The utility of statins in reducing cardiovascular events in patients with coronary artery disease is established. Atorvastatin is a known potent inhibitor of the P-gp system which also interacts with the CYP3A4.9 Simvastatin and lovastatin also interact with the CYP3A4 with only the latter causing P-gp inhibition.10 Pravastatin inhibits the P-gp system without CYP interaction as it is mainly renally cleared.10 There exist reports of myopathy and rhabdomyolysis with the concomitant use of atorvastatin, fluvastatin, lovastatin, pravastatin, or simvastatin along with colchicine.5,10 Similarly, gemfibrozil and fenofibrate may potentiate myopathic effects of colchicine.11 In contrast, rosuvastatin and fluvastatin do not interact with the CYP3A4 or P-gp which theoretically makes them safer options to use with colchicine from a pharmacodynamics standpoint.10 A recent statement from the American Heart Association labels the concomitant use of colchicine and rosuvastatin, fluvastatin, pitavastatin, or pravastatin as reasonable when clinically indicated. In addition, it advocates for dose reductions of colchicine (loading doses of no more than 0.6-1.2 mg and maintenance doses of 0.3-0.6 mg daily) when co-administered with atorvastatin, simvastatin, or lovastatin or in the presence of renal failure with any statin.10
Many antihypertensive medications have the pharmacodynamic potential to interact with colchicine. Verapamil and diltiazem are the most studied drugs in regard to interaction with colchicine as both drugs inhibit the CYP3A4 and P-gp systems.5,12 There are reports of neuromuscular toxicity when these drugs are co-administered with colchicine. Thus a dose reduction of colchicine is advised when utilized in conjunction with verapamil or diltiazem.11,12 Calcium channel blockers as a drug class seem to share similar P-gp inhibitory properties.7 Nicardipine and verapamil are strong inhibitors of the P-gp, while diltiazem and nifedipine cause weaker inhibition.7 In contrast to calcium channel blockers, there is very limited clinical and pharmacodynamic data on other anti-hypertensive drugs. Losartan causes a weak P-gp system inhibition and has only a small affinity for the CYP3A4 system.7,13 No clinical reports of toxicity exist when losartan is co-administered with colchicine. Nonetheless, valsartan and candesartan are considered safer options as they are not metabolized via the CYP pathway.13 With regard to angiotensin-converting enzyme inhibitors (ACEI), there exist only pharmacodynamic data on captopril, which is defined as a weak P-gp inhibitor.7 Finally, among beta blockers, carvedilol is a strong P-gp inhibitor along with bisoprolol and propranolol, while metoprolol and atenolol do not interact with the system.7
Other cardiovascular medications that have the potential to interact with colchicine include: amiodarone, dronedarone, digoxin, ranolazine, and warfarin. Amiodarone and dronedarone are regarded as potent P-gp inhibitors. In contrast, sotalol does not inhibit the P-gp system.7 Ranolazine, a CYP3A4 and P-gp inhibitor, can lead to toxicity when used in combination with colchicine.10,11 Digoxin has been also associated with cases of myopathy and rhabdomyolysis when used with adjunctive colchicine.11 Finally, warfarin is both a substrate and inhibitor of the P-gp transported in the hepatocytes while the newer anticoagulants are only substrate of the P-gp system without causing inhibition.7
There are currently no studies evaluating the clinical impact of the aforementioned interactions of colchicine in cardiovascular medicine. Current FDA recommendations contraindicate the co-administration of colchicine with either a strong CYP3A4 or P-gp inhibitor in patient with renal or liver dysfunction.6 It also suggests that the use of colchicine with moderate or strong CYP3A4 or P-gp inhibitors should be avoided,6 unless there is no alternate regimen and the benefits outweighs the risks. When colchicine is co-administered with CYP3A4 and/or P-gp inhibitors, colchicine dose reduction and/or cautious monitoring are recommended.12 A similar reduction in dosage must be used in the elderly and in those with severe renal (CrCl <30) or liver dysfunction.11 Nonetheless, a dose reduction strategy decreases the incidence of adverse effects, yet it does not eliminate them.1
The use of colchicine in cardiovascular medicine is expanding with promising results beyond the management of acute and chronic pericardial diseases. Cardiologists must be aware of the potential drug-drug interactions and toxicity with this agent. In the setting of an established indication for colchicine, clinicians should make every possible effort to favor, when appropriate, ancillary cardiovascular drug with no or minimal interaction with the CYP3A4 and P-gp When no safer regimen exists, a colchicine dose reduction strategy with close monitoring must be implemented. Nonetheless, it is evident that further research is needed to determine the magnitude and clinical significance of these pharmacodynamics interactions.
References
- Hemkens LG, Ewald H, Gloy VL, et al. Colchicine for prevention of cardiovascular events. Cochrane Database Syst Rev. 2016:CD011047.
- Verma S, Eikelboom JW, Nidorf SM, et al. Colchicine in cardiac disease: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2015;15:96.
- Slobodnick A, Shah B, Pillinger MH, Krasnokutsky S. Colchicine: old and new. Am J Med 2015;128:461-70.
- Chen K, Schenone A, Borges N, Militello M, Menon V. Teaching an old dog new tricks: colchicine in cardiovascular medicine. Am J Cardiovasc Drugs 2017. [Epub ahead of print]
- Mutual Pharmaceutical Company Inc. Pharmacology Reviews. FDA/Center for Drug Evaluation and Research. 2009.
- FDA. Information for Healthcare Professionals: New Safety Information for Colchicine (marketed as Colcrys). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsand
Providers/DrugSafetyInformationforHeathcareProfessionals/ucm174315.htm. 2013. Accessed March 10, 2016. - Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol 2013;61:2495-502.
- Deftereos S, Giannopoulos G, Papoutsidakis N, et al. Colchicine and the heart: pushing the envelope. J Am Coll Cardiol 2013;62:1817-25.
- Davis MW, Wason S. Effect of steady-state atorvastatin on the pharmacokinetics of a single dose of colchicine in healthy adults under fasted conditions. Clin Drug Investig 2014;34:259-67.
- Wiggins BS, Saseen JJ, Page RL, et al. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2016;134:e468-95.
- Mutual Pharmaceutical Company Inc. Colcrys® (colchicine) oral tablets: US prescribing information.
- Terkeltaub RA, Furst DE, Digiacinto JL, Kook KA, Davis MW. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum 2011;63:2226-37.
- Flockhart D, Tanus-Santos J. Implications of cytochrome P450 interactions when prescribing medication for hypertension. Arch Intern Med 2002;162:405-12.

