The Nuts and Bolts of RPM: Is it Right For Your Practice?

Cardiology has been a field of early adopters of technology for many years and digital devices are no exception (Wassif, 2019). Cardiologists interested in exploring digital devices and wearable technology for remotely following chronic cardiovascular diseases such as congestive heart failure (CHF), coronary artery disease (CAD) and vascular disease is of no surprise. (Chatterjee, 2017) COVID-19 clearly highlighted the need to reduce in-person office visits for patients in order to reduce exposure to the virus. Innovative strategies for evaluating patients through telemedicine increased exponentially in 2020. Along with the development of wearable and other digital devices which examine and assess patients from afar, sophisticated computer platforms allowing real time measurement of trends utilizing augmented intelligence (AI) of this data have increased dramatically. These data, collected away from a clinic or hospital, are considered forms of remote physiologic monitoring (RPM). (Vegesna, 2017) By now, you have undoubtedly been approached by a myriad of companies offering to provide your practice with a "no hassle" RPM solution promising to create significant income and improved patient care through monitoring services. This article will help you answer the question, "Is my practice ready for RPM?" It is not a technical comparison of commercial telemonitoring devices.
What Conditions Can You Monitor?
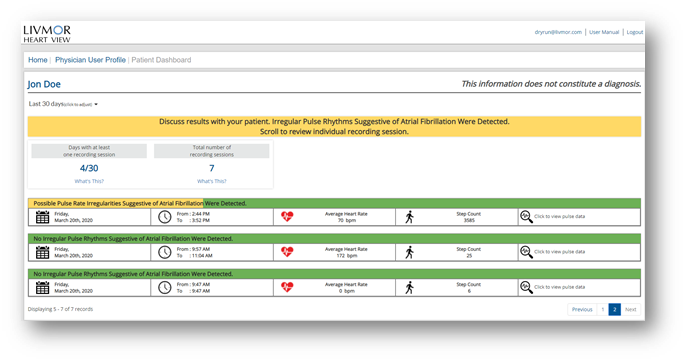
Chronic cardiac conditions, like hypertension (HTN) where home blood pressures (BP) are often more accurate than in-office measurements or CHF where daily changes in vital signs and symptoms may give early signals of patient deterioration seem well-suited to remote monitoring. Tracking daily BP, weight, oxygen saturation, sleep patterns, and heart rhythm changes utilizing wearable sensors may uncover acute disease worsening. The hope is to identify patients in need of urgent office provider attention, potentially reducing emergency room visits and hospitalizations. (Schreiber, 2014). These are in contrast to traditional monitors such as a Holter or Ziopatch, which are used to detect symptomatic events, including supraventricular tachycardia, heart block and vasovagal changes. Several studies comparing traditional office-based monitoring vs. telemonitoring, which forms a collaboration between patient, monitoring team and provider, have documented improved outcomes and health care costs (Ding, 2020). Other chronic conditions which may be monitored include CAD through high sensitivity heart rate variability sensors, vascular disease and diabetic foot ulcers using temperature sensor socks or pads like Siren or Podimetrics, and other conditions such as diabetes utilizing continuous glucose monitors and newer implantables such as the Senseonics device. In general, when considering RPM, think of useful vital signs which would change your treatment algorithm for a patient if you had the information during a regular office visit. For diabetic patients, continuous glucose monitoring devices have provided real time feedback for patients and have proven reduced hospitalizations for ketoacidosis if caught early. (Monostra, 2021) Additionally, hypertensive patients transmitting regular remote BP monitoring with trend analysis, avoiding erroneous "white coat" BP values, can assist clinicians with better BP control and dosing strategies. Patients with high suspicion for arrythmias, particularly intermittent atrial fibrillation (AFib) who remain undiagnosed despite telemetry monitoring, could benefit from continuous AFib detection watches. As more devices are developed, it makes sense that a tailored approach to monitoring a disease state would come from a single agnostic platform transmitting vital physiologic data from several devices which collectively increase the clinician's remote monitoring capability and preventative intervention prior to costly ER visits or hospitalizations.
How Do I Start an RPM Program?
How then can practices seeking to be early adopters proceed? First, select a patient group that is prevalent in your practice. Perhaps difficult to control hypertensives or a heart failure (HF) group that contains high clinical risk or high utilization individuals. Second, develop criteria to identify the patients to be offered monitoring and develop a written adherence contract with patients to acquire data, and track clinical, utilization or cost outcomes. Third, apply this framework to a small, highly selected, high-yield subgroup of patients giving you early experience of an end-to-end solution from diagnosis to intervention to outcome, and limit your initial RPM costs. Your patient selection and device-use protocols should have a high positive predictive value, and the monitor-derived diagnosis or finding should be linked to interventions that improve outcomes or reduce costs.
Which Devices Should You Choose?
The number of wearable devices has exploded in the past five years. Some are coupled to a proprietary mobile application and others are part of a digital solution with a platform able to communicate with your health care provider and the electronic health record (EHR) through back-end application programming interfaces. Many devices are available directly to consumers and are not approved by the U.S. Food and Drug Administration (FDA). Since many are not considered medical grade (but are vastly popular) we focus on reliable devices with accurate data confirmed by the rigorous approval methods of the FDA (Sanders, 2020). From a clinician's standpoint there are several basic features which should be present when considering adoption of physiologic monitoring devices. Is the monitoring device part of a platform and does it not rely on a patient's accessibility to WiFi? Is the platform secure and does it protect patient confidentiality? Does it include disease based physiologic measurements like BP cuffs, weight scales, and oxygen sensors for CHF or does the digital device measure a single parameter, like sleep patterns, etc. which requires a proprietary application without integration into the greater EHR or other devices? It is recommended to consider monitoring solutions instead of single point systems each with individual applications.
Do I Need to Hire More Staff For RPM?
 BioIntelliSense's new BioButton™ for 90-days of continuous wireless temperature and vital signs monitoring on a coin-sized disposable medical device.
BioIntelliSense's new BioButton™ for 90-days of continuous wireless temperature and vital signs monitoring on a coin-sized disposable medical device.Click the image above for a larger view.
Simply answered, no. However, that depends on the volume of patients being followed by RPM services and the number of platforms or applications adopted by the practice or cardiology section. A well-organized team of medical assistants, nurse practitioners (NPs) and physician assistants (PAs) with direct access to physicians can manage several hundred patients safely and with an excellent patient experience. The most important process in a successful RPM program is a specific set of rules for escalation. For instance, just like a Holter or telemetry monitor with multiple hours of normal or unactionable data such as normal sinus rhythm, a single event of non-sustained ventricular tachycardia may require immediate provider review. Best practices include limiting the number of platforms or dashboards to specific disease states which can be easily grouped for review. For instance, we follow CHF patients with a single platform with a BP cuff, weight scale and an oxygen saturation device all connected to a single platform with an easy-to-read dashboard. In addition, escalation rules are set up for abnormal (red) or normal (green) findings on the site, allowing for easy intervention when necessary. The abnormal results are first reviewed by the medical assistants, then escalated to advanced providers (NP or PAs) then and only if serious, elevated to physicians for review. (Mosnaim, 2020) An audit of the system is performed monthly to allow for quality assurance with random patient dashboard reviews. This alleviates the two most common concerns frequently heard from busy cardiologists. First, concern regarding how to manage the large volume of data from RPM and second, the liability of missing important physiologic data. Both are easily handled with the strategies suggested.
Is Anyone Willing to Pay For RPM?
The question that inevitably seems to get asked whenever incredible innovations are introduced in health care is, "Who's going to pay for it?" Adoption of novel solutions often precedes payment in cardiovascular disease, but often with proof, reimbursement often catches up to innovation. RPM, on the other hand, already has approved CPT payment codes from Medicare and commercial payors that have been established since January 2019. (Mechlai, 2021) In 2020, the Centers for Medicare and Medicaid Services (CMS) doubled down by stating they would pay for several new CPT codes that not only reimburse providers for various types of care they may already be providing in the interest of keeping patients healthier and out of the hospital, but they also open new potential revenue streams to help ease the transition into value-based care.
The Economics of Coding For RPM:
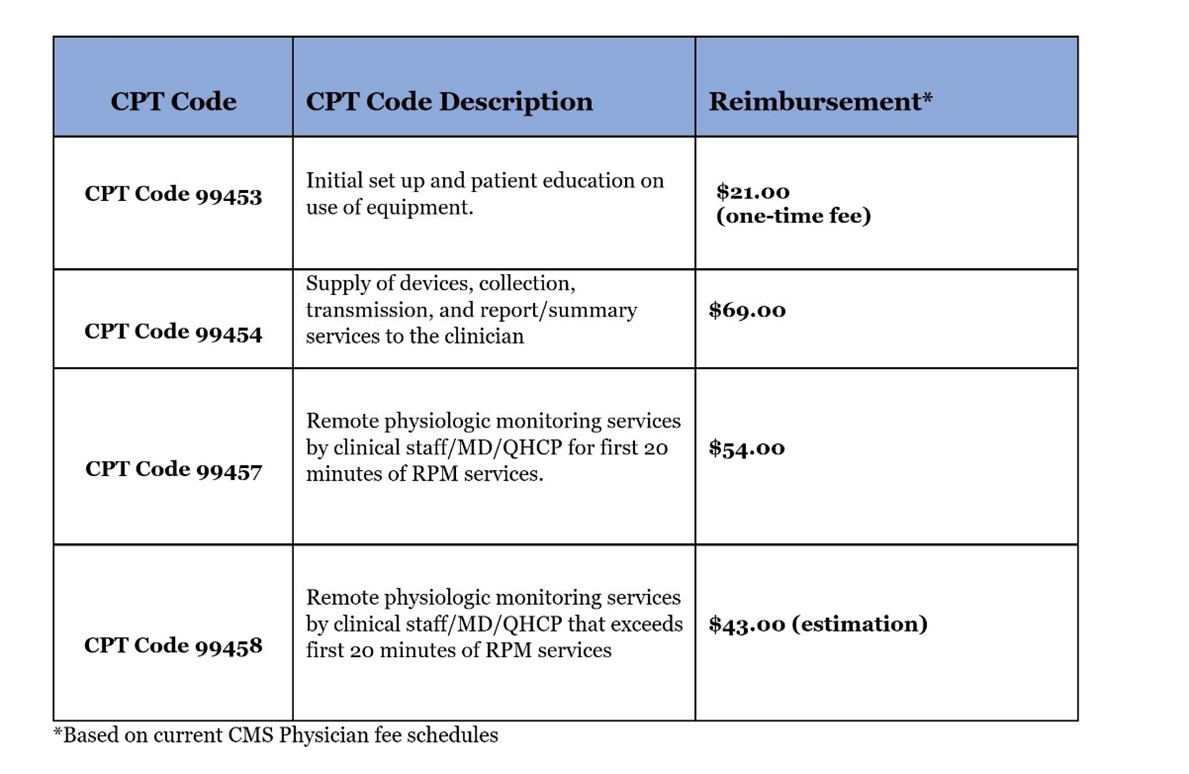
Code 99453 covers the setup of devices in an episode of care and patient education, while code 99454 covers the cost of device(s) with daily recording(s) or programmed alert(s) and can be billed each 30 days. These CPT codes offer reimbursement for providing the patient with a device as defined by the FDA. We believe CMS may further define the device definition in 2020, but it currently remains very broad. Code 99457 covers the first 20-minutes each calendar month of RPM treatment management services, of clinical staff/physician/other qualified health care professional time requiring interactive communication with the patient/caregiver during the month. CMS began paying for CPT code 99458 on Jan. 1, 2020. The new code covers each additional 20 minutes (each calendar month) spent on treatment management services. Another important change that began Jan. 1, 2020, CPT codes 99457 and 99458 will be designated as care management services by CMS, which means they can be furnished under general rather than direct supervision of the billing provider (Tashnek, 2021). The net effect is that the physician or other qualified health care professional supervising the delivery of RPM services does not have to be located at the same site as the clinical staff actually delivering them.
Key Considerations For Selecting an RPM Partner:
With so many companies now proclaiming themselves as having the "the best RPM solution on the market," how do you choose the one that is right for your heart program? Here are some questions to try and objectively compare your options:
- First, decide which remote monitoring services are the most important for you to offer. As noted earlier in the article, BP cuffs and scales are appropriate for a CHF targeted patient population but that may differ if you are targeting other chronic conditions. Ideally you would pick an RPM partner that has a multitude of device options so you can expand your options through a single vendor platform.
- Second, pay close attention to ease of use and setup. How much work will be required to start the RPM program and maintain it? Does it fit easily into your clinical workflows or can it be customized to do so? Functionality will play a large role in its usefulness and adoption by both patients and your staff.
- Third, get a clear understanding of the financial dynamics of the RPM devices. Do you need to purchase them and then distribute them to patients? Do patients purchase or lease them directly from the RPM vendor? Related to this, who provides training and support for the RPM devices if they there are issues – you or the RPM vendor? This will determine the overall fees you pay and can vary widely depending on the RPM vendor and approach taken.
- Fourth, what are the revenue cycle implications of your RPM approach? How will your RPM activity be coded and billed – by your organization or the RPM vendor partner?
- Fifth, what is the technological approach of the RPM devices – cellular, digital or a combination depending on the device?
- Sixth, if you are currently offering chronic care management (CCM) services, how does the RPM strategy integrate with your CCM program? Is the RPM data consolidated with your CCM data giving you an integrated picture of patient status or are they totally separate?
In general, you will likely end up comparing RPM options that are either "full-service" where you contract with an RPM company who typically handles the distribution, management and provides you with their own proprietary website to view device activity. The other alternative is a "self-managed" program where you own and distribute devices, provide technical support and utilize your own (or a third party) RPM monitoring software to manage device data. Both have their advantages and disadvantages. Finally, RPM is a dynamic and rapidly evolving market with significant investments being made in new product development. What is best in class today may not be best in class in six months. Make sure you understand the likelihood of your RPM partner to evolve and innovate or have the option to move to other partners and devices as they appear on the market.
Conclusion:
The enthusiasm from digital health companies to develop wearables and sensor devices has been fueled by the enormous investment from venture capital firms over the last five years. The tech companies responsible for the development have followed the traditional motto of making devices and "failing fast" in the consumer market. More recently, clinicians have questioned the validity of device data and demanded higher standards of sensitivity and specificity, including FDA approval. Although CMS has approved RPM codes, there exist challenges in developing a cogent strategy at the practice level as to implementation and device choice. Systems which represent solutions as opposed to only single vital sign parameters will likely be the winners in this race to untether medicine from traditional bricks and mortar office or hospital visits to a more decentralized monitoring program.
The ACC has relationships with BioIntelliSense and cliexa. Learn more about the ACC Innovation Program.
Bibliography:
Wassif HS. (2019). The Innovation Cycle: How Do Adoption and Abandonment Feed It? Cardiology Magazine.
Chatterjee NA, Singh JP (2017). Making Sense of remote monitoring studies in Heart Failure. Eur Heart Journal, 38, 2361.
Mechlai K, Smith N, Stern AD, Cramer DB (2021). Remote Patient Monitoring-Overdue or Overused? The New England Journal of Medicine. N Engl J Med 2021 Apr 15;384(15):1384-1386 doi: 10.1056/NEJMp2033275.
Ding H , Chen SH , Edwards I , Jayasena R , Doecke J , Layland J , Yang IA , Maiorana A. Effects of Different Telemonitoring Strategies on Chronic Heart Failure Care: Systematic Review and Subgroup Meta-Analysis: Effects of Different Telemonitoring Strategies on Chronic Heart Failure Care: Systematic Review and Subgroup Meta-Analysis. J Med Internet Rs 2020:22 (11): e20032 doi:10.2196/20032
Abraham WT, Adamson PB, Hasan A, Bourge RC, Pamboukian SV, Aaron MF, Raval NY. Safety and accuracy of a wireless pulmonary artery pressure monitoring system in patients with heart failure. Am Heart J 2011; 161:5 58-66.
Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System J Am Coll Cardiol 2013; 61:1571–6
Dhruva SS, Krumholz HM. Championing Effectiveness before Cost-Effectiveness JACC Heart Fail. 2016 May ; 4(5): 376–379. doi:10.1016/j.jchf.2016.02.001.
Sandhu AT, Goldhaber-Fiebert JD, Owens DK, Turakhia MP, Kaiser DW, Heidenreich PA. Cost-Effectiveness of Implantable Pulmonary Artery Pressure Monitoring in Chronic Heart Failure JACC Heart Fail. 2016 May ; 4(5): 368–375. doi:10.1016/j.jchf.2015.12.015
Rainmaker C. https://www.dcrainmaker.com/2020/09/apple-watch-series-6-first-run-accuracy-spo2-sensor-data.html posted Sept 19, 2020 downloaded 4/30/2021 2 PM
Monostra M (2021). Fewer Hospitalizations, but higher total health costs 1 year after CGM initiation. Endocrinology.
Mosnaim G, Stempel H, Van Sickle D, Stempel DA (2020). The Adoption and Implementation of Digital Healthcare in the Post Covid 19 Era. J Allery Clin Immun Pract, 8(8), 2484-2486.
Perakslis E, Ginsburg G. (2020). Digital Health-The Need to Assess Benefits, Risks and Value. JAMA, E1-E2. JAMA. 2021;325(2):127-128. doi:10.1001/jama.2020.22919
Peretz D, Arnaert A, Ponzoni NN (2016). Determining the Cost of Implementing and Opeating a Remote Patient Monitoring Programme for the Elderly with Chronic Conditions: A systematic review of economic evaluations. Journal of Telemedicine and Telecare.
Sanders SF, Stern AD, Gordon WJ. (2020, July 2). Monitoring Tech Part of Everyday Health Care. Harvard Business Review.
Schreiber D, Sattar A, Drigalla D, Higgins S (2014). Ambulatory Cardiac Monitoring for Discharged Patients with Possible Cardiac Arrythmias. West J Emerg Med, 15, 194-198.
Tashnek D. (2021, Feb 3). CMS Announces 3 Significant Corrections to Remote Patient Monitoring. Becker's Hospital Review.
Vegesna A, Tran M, Angelaccio M, Arcona S (2017). Remote Monitoring via Non-Invasive Digital Technologies: A Systematic Review. Telemed J E Health, 23, 3-17.
Jia X, Kohli P. (2020). Telehealth and Cardiovascular Disease Prevention: A Discussion of the Why and the How. ACC Online Journal Expert Analysis.
ACC Members, discuss
this on Member Hub.
This content was developed independently from the content developed for ACC.org. This content was not reviewed by the American College of Cardiology (ACC) for medical accuracy and the content is provided on an "as is" basis. Inclusion on ACC.org does not constitute a guarantee or endorsement by the ACC and ACC makes no warranty that the content is accurate, complete or error-free. The content is not a substitute for personalized medical advice and is not intended to be used as the sole basis for making individualized medical or health-related decisions. Statements or opinions expressed in this content reflect the views of the authors and do not reflect the official policy of ACC.