More High-Risk Device-Related Thrombi With Watchman Than Amulet Device For LAAO
More patients who received the Watchman 2.5 device compared with the Amulet occluder device for left atrial appendage occlusion experienced high-risk device-related thrombi (DRTs), according to a study published April 9 in JACC: Clinical Electrophysiology. Additionally, patients with high-risk DRTs, compared with low risk or none, had a higher rate of the composite of stroke, systemic embolism or cardiovascular death at five years.
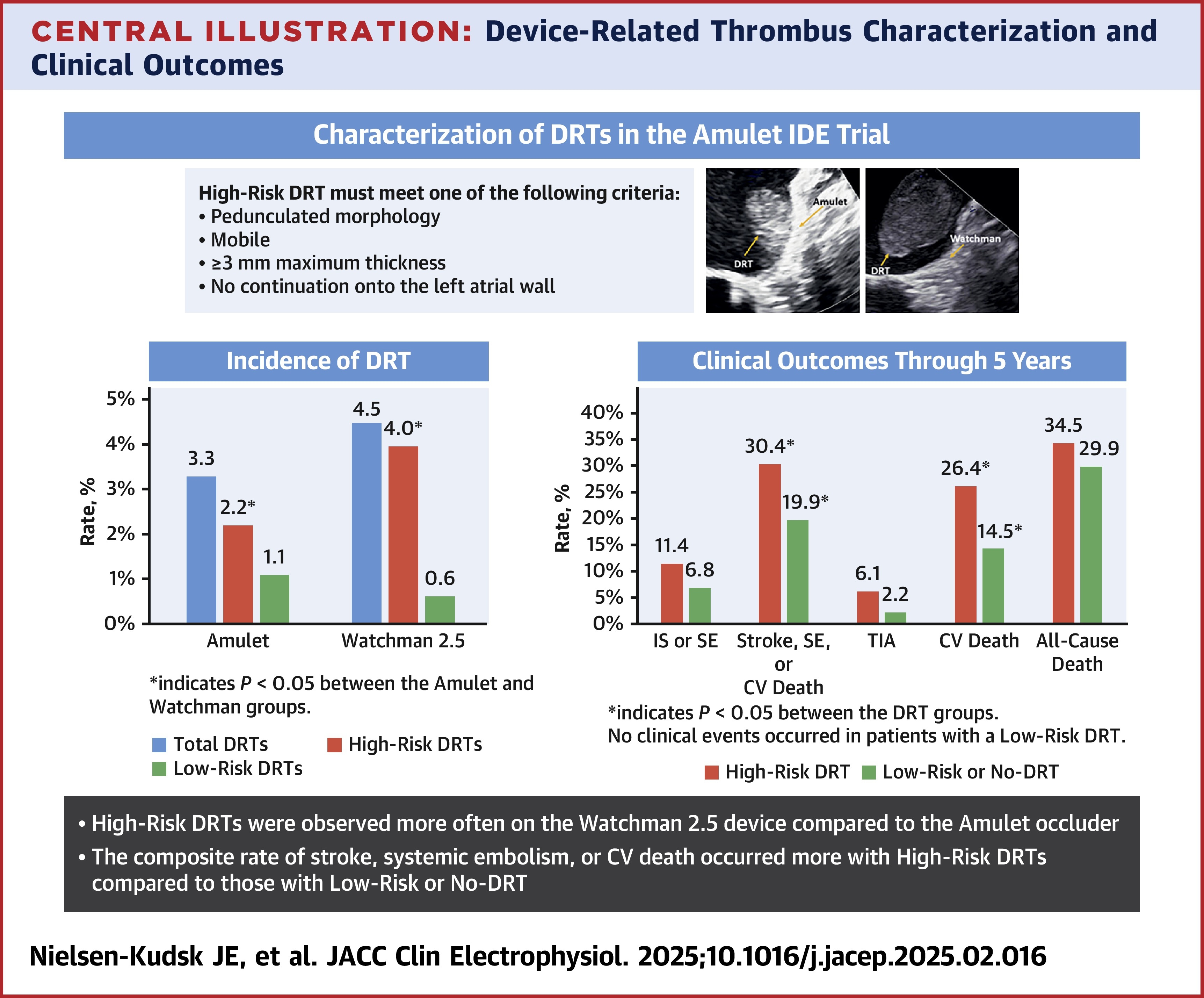
In the Amulet IDE trial, 1,788 patients with nonvalvular atrial fibrillation (AFib) were successfully implanted with either a single-lobe Watchman 2.5 (n=885) or a dual-seal Amulet occluder device (n=903). Of these, a DRT was observed in 40 patients (4.5%) in the Watchman group and 30 patients (3.3%) in the Amulet group.
In this analysis, researchers categorized DRTs, diagnosed using transesophageal echocardiography, as "high-risk" if they were pedunculated, mobile, ≥3 mm thick or without continuation onto the left atrial wall. Around 30% of DRTs were found around the screw hub of both devices, and the most common site for a high-risk DRT, accounting for more than 40%, was at the superior area of the device surface near the left upper pulmonary vein ridge. Most high-risk DRTs were identified beyond 45 days of implantation.
Patients with high-risk DRTs were more likely to be older, female, in AFib at the time of the procedure, have a history of renal or urinary disorder, and have a higher CHA2DS2-VASc score.
Results at 12 months showed there were significantly more high-risk DRTs with the Watchman 2.5 than the Amulet device (4.0% vs. 2.2%; p=0.030). More of the low-risk DRTs resolved (12 of 15) than the high-risk DRTs (21 of 55).
Looking at clinical outcomes, at five years, 30.4% and 19.9% of the high-risk and low-risk/no risk DRT patients experienced the composite endpoint of stroke, systemic embolism or cardiovascular death (HR, 1.74). Cardiovascular death occurred in 26.4% and 14.5% of the groups (HR, 2.09). No significant difference was observed in clinical event rates in relation to the device implanted.
"The Watchman's single-lobe design may create conditions more conducive to thrombus formation by not conforming as well to varied morphologies of the LAA, potentially leaving small gaps where blood can pool and clot," write Jens Erik Nielsen-Kudsk, MD, et al., when comparing the two devices. "Conversely, the Amulet's dual-seal design has shown to be successfully implanted in complex anatomies with its disc and lobe components, providing a more effective LAA closure and thereby potentially reducing the risk of thrombus formation." They add that the Watchman 2.5 is not the most recent generation of the device and a newer model may yield different outcomes.
Noting the longer timespan in the identification of high-risk DRTs, the authors also suggest scheduling an imaging follow-up time beyond the standard 45 days.
Clinical Topics: Arrhythmias and Clinical EP, Noninvasive Imaging, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Echocardiography/Ultrasound
Keywords: Electrophysiology, Atrial Fibrillation, Thrombosis, Thromboembolism, Atrial Function, Left, Echocardiography, Transesophageal
< Back to Listings

