The Extravascular Implantable Cardioverter-Defibrillator: A Promising Novel Device
Quick Takes
- The extravascular implantable cardioverter-defibrillator (EV-ICD) effectively terminates ventricular arrhythmias and can be implanted safely.
- The substernal position of the EV-ICD lead allows for antitachycardia pacing and pause prevention pacing capabilities.
- Improvements are needed to decrease the rate of inappropriate therapies delivered by the EV-ICD.
Transvenous implantable cardioverter-defibrillators (ICDs) are the reference standard for preventing sudden cardiac death (SCD) but have risks associated with intravascular lead placement.1 ICD technology has evolved from the epicardial to the transvenous era to extravascular innovations that avoid vascular complications, including cardiac perforation, venous obstruction, increased infection rates, and tricuspid regurgitation. Among these innovations is the established subcutaneous implantable cardioverter-defibrillator (S-ICD), which has its own limitations, such as the absence of pacing capability, high defibrillation threshold, large device size, and reduced projected battery longevity.2 The extravascular implantable cardioverter-defibrillator (EV-ICD) is a Food and Drug Administration (FDA)–approved emerging technology that offers another option for an implantable system that avoids the vascular risks associated with transvenous ICDs. It is unique in that it is composed of a lead that is tunneled posterior to the sternum rather than anterior (Figure 1).3 The early studies have provided compelling evidence supporting the EV-ICD as a valid alternative to the more established transvenous ICD and S-ICD systems.4 However, data from these studies have also identified several areas that require improvement to optimize the performance and efficacy of the EV-ICD.4-6
Figure 1: The EV-ICD System
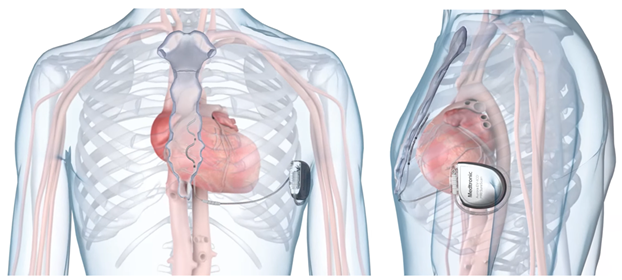
Anteroposterior (left) and lateral (right) views of the implantable EV-ICD. © 2024 Medtronic. All rights reserved. Used with the permission of Medtronic.
EV-ICD = extravascular implantable cardioverter-defibrillator.
The EV-ICD has multiple advantages and is both safely implantable and effective at preventing SCD (Figure 2). The Extravascular ICD Pivotal Study data showed that the rates of successful defibrillation at implantation (98.7%) and at 6-month follow-up (100%) are greater than those for transvenous ICDs (90.5-93%) and similar to those for S-ICDs (100%).4 The data also showed that rates of successful defibrillation of discrete spontaneous events are 100%, with first shock efficacy at 78%, the latter being lower than that of current transvenous ICDs and S-ICDs.4 Induced ventricular tachycardia (VT) or ventricular fibrillation (VF) was detected in all patients during implantation testing, demonstrating that extravascular sensing and detection are reliable. From a safety perspective, EV-ICDs implanted by trained providers had 92.6% freedom from system-related and procedure-related complications, which is in line with data from transvenous ICD and S-ICD studies; notably, the number of EV-ICD systems that required removal due to infection was similar to that for S-ICDs.4 The efficacy and safety of the EV-ICD were achieved without sacrificing efficiency; the Extravascular ICD Pivotal Study data demonstrated that procedure times for the EV-ICD are similar to those for early S-ICDs and that revision frequency is better than that for transvenous ICDs.4 Additionally, by positioning the lead in the substernal space (closer to the myocardium and without the sternum as a barrier), these devices achieve lower defibrillation and pacing energy requirements than do S-ICDs. This optimization of energy delivery with a maximum shock energy of 40 J enhances device durability (projected 60% longer battery life than that for the S-ICD) and reduces the need for frequent generator replacements (up to two fewer replacement surgeries over a modeled patient lifetime).4 The lower defibrillation energy requirement also enables the EV-ICD generator (33 cm3) to be smaller than the S-ICD generator and similar in size to transvenous ICD generators. Finally, the pacing capabilities of the EV-ICD allow for antitachycardia pacing (ATP), pause-prevention pacing at 40 bpm for up to 30 sec, and postshock pacing at the same rate as pause-prevention pacing.3,4 The Extravascular ICD Pivotal Study data showed that 70% of arrhythmic episodes are terminated via ATP, allowing for fewer shocks needed to treat ventricular arrhythmias; in the study, 33 shocks were avoided in seven patients.4 These features enable the EV-ICD to have multiple programable VT zones similar to the transvenous ICD.
Figure 2: EV-ICD Pros and Cons
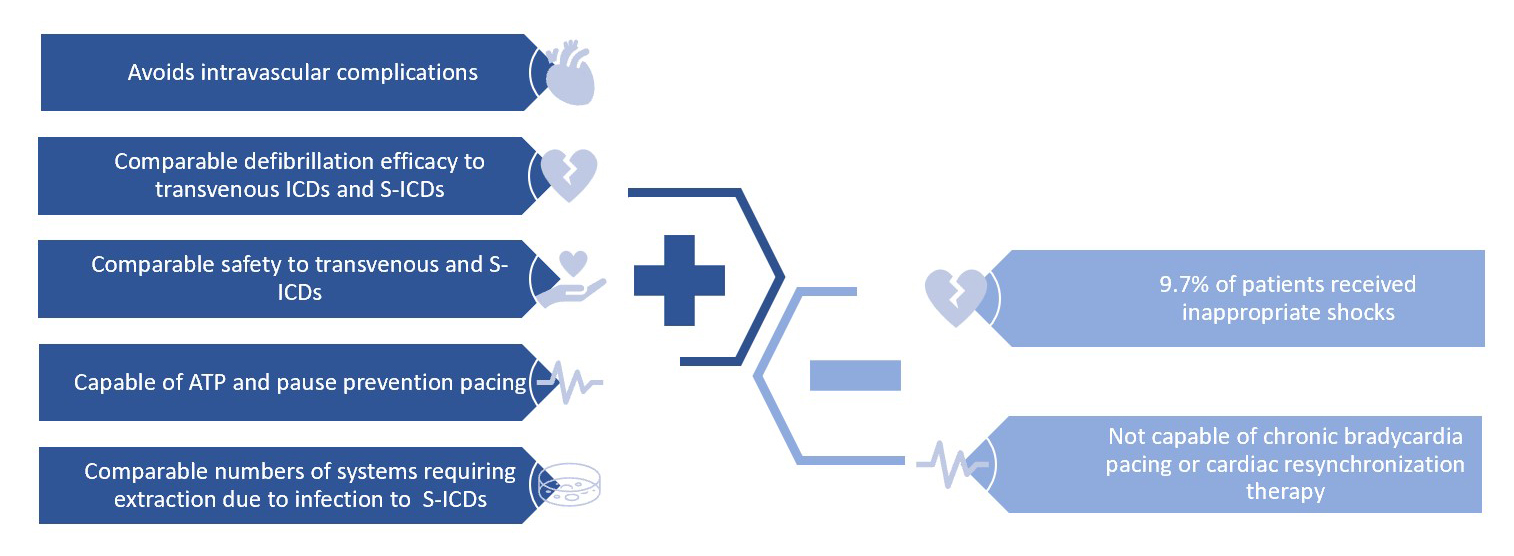
ATP = antitachycardia pacing; EV-ICD = extravascular implantable cardioverter-defibrillator; ICD = implantable cardioverter-defibrillator; S-ICD = subcutaneous implantable cardioverter-defibrillator.
Despite its advantages, the EV-ICD is not without limitations, as depicted in Figure 2. One primary concern revolves around the occurrence of inappropriate therapies, highlighting the significance of lead positioning to mitigate P-wave oversensing issues. In the Extravascular ICD Pivotal Study, P-wave oversensing had an incidence of 9.7%, necessitating lead repositioning during implantation, which resulted in a one-third reduction in patients receiving inappropriate shocks.4 To further address this issue, several novel features have been integrated into the EV-ICD system to decrease the occurrence of inappropriate shocks. Among these features, the P-wave oversensing (PWOS) algorithm stands out and is projected to reduce inappropriate detection by 29%.5 The PWOS algorithm identifies alternating low amplitudes of the P waves and high amplitudes of the R waves, aiding in accurate rhythm discrimination.5
Additionally, various strategies have been incorporated into the VT–supraventricular tachycardia (SVT) discriminators to enhance specificity and sensitivity. For instance, the morphology noise feature distinguishes between VF and noise on the sensing channel by discriminating VF from noise on the far-field channel, whereas adjustments to the required wavelet mismatch improve the sensitivity of SVT discriminators (3:8 wavelet match >61% in EV-ICDs vs. >70% in transvenous ICDs). Moreover, comparing the R-wave width between the sinus and tachycardia rhythms and matching overall wavelet morphology further enhances discrimination capabilities. These advancements aim to offset the risk of inappropriate therapy due to respirophasic R-R variability inherent to the substernal position of the lead. By implementing these features, the EV-ICD strives to optimize therapy delivery while minimizing the occurrence of inappropriate shocks, thereby improving patient outcomes in clinical practice. Another limitation is that patients requiring cardiac resynchronization or chronic bradycardia pacing are currently not candidates for the EV-ICD and some may experience a pacing sensation despite the lower pacing energy, making chronic pacing undesirable.6 Additionally, certain patient populations are excluded from EV-ICD implants, such as those with previous sternotomy, previous surgery disrupting the pericardium or tissue outside the pericardium, previous chest radiation, pericardial disease, or mediastinitis.4 Lastly, the implementation of EV-ICD technology necessitates a partnership between electrophysiologists and cardiac surgeons and entails a learning curve, which is yet to be fully defined as the device becomes more widely available.
In summary, the EV-ICD is a promising new device that does not require intravascular access, maintains effective defibrillation, and has ATP and pause-prevention pacing capabilities. The major concern is inappropriate shocks, and improvements are in process to reduce these unwanted events. Looking ahead, several improvements and iterations of extravascular ICDs are anticipated. Among these advancements is the introduction of a parasternal DF4 lead positioned in the anterior mediastinum and atop the pericardium. This innovative design allows for connection to commercially available transvenous ICD generators placed in the pectoral area, potentially combining the benefits of both extravascular and transvenous systems.7
References
- Knops RE, Olde Nordkamp LRA, Delnoy PHM, et al.; PRAETORIAN Investigators. Subcutaneous or transvenous defibrillator therapy. N Engl J Med 2020;383:526-36.
- Nso N, Nassar M, Lakhdar S, et al. Comparative assessment of transvenous versus subcutaneous implantable cardioverter-defibrillator therapy outcomes: an updated systematic review and meta-analysis. Int J Cardiol 2022;349:62-78.
- Thompson AE, Atwater B, Boersma L, et al. The development of the extravascular defibrillator with substernal lead placement: a new frontier for device-based treatment of sudden cardiac arrest. J Cardiovasc Electrophysiol 2022;33:1085-95.
- Friedman P, Murgatroyd F, Boersma LVA, et al.; Extravascular ICD Pivotal Study Investigators. Efficacy and safety of an extravascular implantable cardioverter-defibrillator. N Engl J Med 2022;387:1292-302.
- Swerdlow CD, Zhang X, Liu Y, et al. CI-452770-4 PERFORMANCE OF A NOVEL P-WAVE OVERSENSING REJECTION ALGORITHM TO REDUCE INAPPROPRIATE DETECTIONS IN THE EXTRAVASCULAR ICD. Heart Rhythm 2023;20:S87.
- Sholevar DP, Tung S, Kuriachan V, et al.; SPACE Study Investigators. Feasibility of extravascular pacing with a novel substernal electrode configuration: the Substernal Pacing Acute Clinical Evaluation study. Heart Rhythm 2018;15:536-42.
- Burke MC, Knops RE, Reddy V, et al. Initial experience with intercostal insertion of an extravascular ICD lead compatible with existing pulse generators. Circ Arrhythm Electrophysiol 2023;16:421-32.
Clinical Topics: Arrhythmias and Clinical EP, Implantable Devices, SCD/Ventricular Arrhythmias
Keywords: Defibrillators, Implantable
