Increasing the Number of Women and Underrepresented Groups in Clinical Trials
Quick Takes
- Clinical trials are not inclusive and do not represent our diverse patient populations living with CVD, but they are nonetheless often used to create guidelines and dictate clinical care including among populations in which there is limited or no knowledge regarding treatment effects and outcomes.
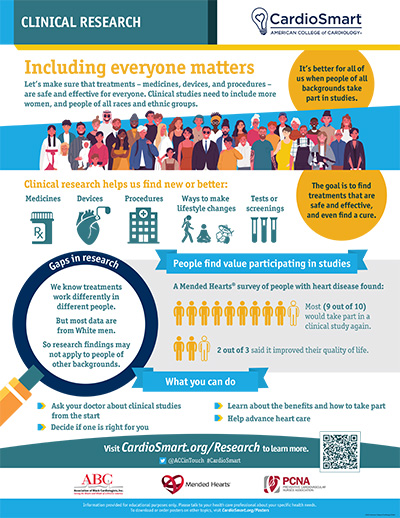
- Physicians can ask patients if they are interested in clinical trials, educate patients about the benefit in participation, and emphasize how participation can lead to improved care. ACC's CardioSmart has partnered with the Association of Black Cardiologists, Mended Hearts, and the Preventive Cardiovascular Nurses Association on an infographic for use at the point of care to support these conversations with our patients. Also, Mended Hearts has additional resources, including discussion guides.
- Overall, enrollment in clinical trials is a team effort. During recruitment, physicians and research staff must address barriers to participation, such as financial concerns, talk about health and safety risks, and allow family members to be engaged in the decision-making process.
Cardiovascular disease (CVD) is the leading cause of death worldwide, with a growing prevalence among women, Hispanics, and Black people. These populations are underrepresented in the clinical trials that guide our daily medical practice.1,2 Historically, CVD has been viewed as a predominately male disease, but epidemiological studies show a greater incidence of CVD among women, especially in postmenopausal age groups.3,4 Additionally, women have greater rates of mortality from CVD in comparison to men.1 The lack of diversity in clinical trials and their adverse effects is not limited to women, as trials underrepresent people of diverse backgrounds, including racial and ethnic minority groups, and sexual minority groups including transgender and non-binary communities.5
According to the 2020 Census Bureau's Population Estimation Program, the United States (US) racial composition was predominately 59% White (non-Hispanic), 18.9% Hispanic, 13.6% Black.6 This illustrates an increase in people who are Hispanic and Black since 2010, with more than 50% of the population expected to be other than non-Hispanic White by 2045.6,7 Despite these growing trends, only 5% and 1% of cardiovascular clinical trial subjects enrolled have been Black and Hispanic, respectively.7 Black people are known to be disproportionately affected by CVD.8,9 Risk for CVD is also affected by the social constructs of race as typical risk factors – smoking, hypertension and diabetes – are known to be more prevalent.8,9 CVD outcomes illustrate racial disparities where there is a steeper decline in mortality rates from myocardial infarction in White than Black populations.10,11 Despite advances in medical treatment, women and other historically marginalized groups paradoxically continue to experience poorer outcomes, in part due to a lack of representation in clinical trials.8,11,12
Clinical trials that are not inclusive and do not represent our patient population living with the disease are nonetheless, often used to create guidelines and dictate clinical care, which ultimately affects CVD outcomes.7,9 Important biological differences distributed by age, sex, gender, race and ethnicity can lead to differences in disease presentation, pharmacokinetics, and response to interventions.4,9,10 Women and men were thought to have similar pathophysiology in the manifestation of heart disease; however, as we increase the number of women in clinical trials, we are learning that women are more complex physiologically, and their degree and distribution of CVD may vary.3,8,12 Younger women may have variable presentations and manifestations of CVD, such as myocardial infarction with nonobstructive coronary arteries, certain cases of spontaneous coronary artery dissection, postpartum cardiomyopathy, or stress cardiomyopathy, which not only primarily affects women, but are largely understudied.11 Among transgender populations, studies have been predominately observational, and greater research is needed to better understand why there is a greater risk of CVD than the general population.5
There are multiple limiting factors that prevent the participation of historically marginalized groups in clinical trials. Populations, such as pregnant women, have been excluded, when CVD is the leading cause of maternal mortality among non-Hispanic Black people who have the greatest rates of mortality and morbidity during pregnancy and up to one year postpartum.13 Women of childbearing age were historically prohibited from participating in early phase drug trials given the risk of becoming pregnant and causing harm to the mother and/or fetus during the study period.14 As a result, clinical trials have predominately enrolled White males who were younger than 65 years of age. This effectively excludes post-menopausal women, where CVD is the leading cause of death globally.9,11 Efforts have been made at a larger scale, such as the Revitalization Act of 1993, which required the inclusion of both women and members of minority groups in National Institute of Health funded trials, and the Center for Devices and Radiological Health-Health of Women program mandated by the Food and Drug Administration (FDA) in 2022, which aims to enroll more women in trials to illustrate the impact sex and gender have on a woman's overall health.5,7,15 Nonetheless, since these acts have been passed, the representation of women and minorities has only modestly increased in comparison to the disease burden in these populations.10,16
Poor recruitment of people from racial and ethnic minority groups in clinical trials has been limited by the lack of racial reporting, underutilization of medical resources, and hesitation towards the medical system.7,10 Prior trials have not consistently reported race/ethnicity, thus it is unknown whether the study results can be adequately applied to our diverse patient population.10,12,16 Studies used to shape CVD prevention guidelines reported information on race or ethnicity only in one of three trials.12 One study examined all the cardiovascular clinical trials from 1997-2010 and demonstrated that only half of these studies reported information regarding race or ethnicity.9,12 Financial barriers play a large role in participation where patients may be concerned about the direct and indirect cost of participation. Time away from their jobs and caregiving roles can be especially difficult among women and those from backgrounds that have been historically marginalized.9,10 Unfortunately, distrust is a barrier to active participation as studies show patients are concerned about getting inadequate medical care or being viewed as a subject rather than an individual.10,16
Undoubtedly, physicians play a large role in patient recruitment and are considered one of the most trusted sources of information.7,12 Yet many patients don't learn about the clinical trials they may be eligible for from their physicians. In a recent Mended Hearts survey of 1,081 patients with heart disease, 63.7% of patients said they received information about clinical trials from a source other than their doctor, surgeon or hospital. Only about 1 in 10 patients said they were asked to participate in a clinical trial, but of those asked more than half agreed to participate (53.6%). Physicians can ask patients if they are interested in clinical trials, educate patients about the benefit in participation, and emphasize how participation can lead to improved care. ACC's CardioSmart has partnered with the Association of Black Cardiologists, Mended Hearts, and the Preventive Cardiovascular Nurses Association on an infographic for use at the point of care to support these conversations with our patients. Also, Mended Hearts has additional resources, including discussion guides.
Physicians who most resemble underrepresented patient populations need to become leaders in clinical trials as this will help encourage enrollment.11,14 Indeed increasing the diversity of the cardiology workforce as a whole also will help ensure quality patient care. Institutions that predominately serve racial and ethnic minority groups should be asked to serve as clinical trial sites to help increase diverse patient enrollment.9 Overall, enrollment in clinical trials should be a team effort with close collaboration between the clinical investigators and community members to help alleviate resource constraints and provide a greater knowledge about the trial process.9,10 During recruitment, physicians and researcher staff must address financial concerns, health and safety risks, and allow family members to be engaged in the decision making process.7,10,14,17
Physicians hold the ability to change the narrative of clinical trials in the short- and long-term to better represent patient populations who are diverse in sex, gender, race, and ethnicity to ultimately reduce CVD and improve outcomes.
References
- Westerman S, Wenger NK. Women and heart disease, the underrecognized burden: sex differences, biases, and unmet clinical and research challenges. Clin Sci 2016;130:551-63.
- Brothers RM, Fadel PJ, Keller DM. Racial disparities in cardiovascular disease risk: mechanisms of vascular dysfunction. Am J Physiol Heart Circ Physiol 2019;317:H777-H789.
- Dougherty AH. Gender balance in cardiovascular research. Tex Heart Inst J 2011;38:148-50.
- Vitale C, Fini M, Spoletini I, Lainscak M, Seferovic P, Rosano GM. Under-representation of elderly and women in clinical trials. Int J Cardiol 2017;232:216-21.
- Connelly PJ, Marie Freel E, Perry C, et al. Gender-affirming hormone therapy, vascular health and cardiovascular disease in transgender adults. Hypertension 2019;74:1266-74.
- Race and Ethnicity in the United States: 2010 Census and 2020 Census (census.gov.) 2021. Available at: https://www.census.gov/library/visualizations/interactive/race-and-ethnicity-in-the-united-state-2010-and-2020-census.html. Accessed 11/11/2022.
- Clark LT, Watkins L, Piña IL, et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr Probl Cardiol 2019;44:148-72.
- Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation 2017;136:e393-e423.
- Tahhan AS, Vaduganathan M, Greene SJ, et al. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol 2018;3:1011-19.
- Vilcant V, Ceron C, Verma G, Zeltser R, Makaryus AN. Inclusion of under-represented racial and ethnic groups in cardiovascular clinical trials. Heart Lung Circ 2022;31:1263-68.
- Bucholz EM, Krumholz HM. Women in clinical research: what we need for progress. Circ Cardiovasc Qual Outcomes 2015;8:S1-S3.
- Zhang T, Tsang W, Wijeysundera HC, Ko DT. Reporting and representation of ethnic minorities in cardiovascular trials: a systematic review. Am Heart J 2013;166:52-57.
- ACOG Practice Bulletin No. 212: Pregnancy and heart disease. Obstet Gynecol 2019;133:e320-356.
- Michos ED, Reddy TK, Gulati M, et al. Improving the enrollment of women and racially/ethnically diverse populations in cardiovascular clinical trials: an ASPC practice statement. Am J Prev Cardiol 2021;8:100250.
- Cornelison T. FDA Releases CDRH Health of Women Strategic Plan to Better Inform Medical Device Research and Regulation for All Women (fda.gov). 2022. Available at: https://www.fda.gov/news-events/press-announcements/fda-releases-cdrh-health-women-strategic-plan-better-inform-medical-device-research-and-regulation. Accessed 11/22/2022.
- Turner BE, Steinberg JR, Weeks BT, Rodriguez F, Cullen MR. Race/ethnicity reporting and representation in US clinical trials: a cohort study. Lancet Reg Health Am 2022;11:100252.
- Goldfarb MJ, Bechtel C, Capers Q 4th, et al. Engaging families in adult cardiovascular care: a scientific statement from the American Heart Association. J Am Heart Assoc 2022;11:e025859.
Clinical Topics: Cardiovascular Care Team, Prevention, Hypertension, Arrhythmias and Clinical EP, Dyslipidemia, Heart Failure and Cardiomyopathies, Valvular Heart Disease, Vascular Medicine
Keywords: Clinical Trial, Clinical Trials as Topic, Cardiovascular Diseases, Minority Groups, Ethnic Groups, Cultural Diversity, Health Equity, Gender Equity, Sexual and Gender Minorities, Risk Factors, Heart Disease Risk Factors, Myocardial Infarction, Hypertension, Diabetes Mellitus, Cause of Death
< Back to Listings