The Outcomes Registry For Cardiac Conditions in Athletes (ORCCA) Study: Large-Scale Data Capture For the Sports Cardiology and Sports Medicine Communities
Quick Takes
- Large-scale cardiovascular registries are essential in understanding the natural history, risk factors, and management of cardiovascular conditions.
- Outcomes data among athletes with suspected or high-risk cardiac conditions remain limited, and contemporary guidelines are primarily based on expert opinion.
- The primary goal of the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) study is the assessment of adverse cardiovascular outcomes among competitive athletes with underlying cardiac conditions.
Introduction
Prospective large-scale population cohort studies and clinical registries have played a pivotal role in our understanding of the natural history, risk factors, and management of cardiovascular diseases (CVD). Starting with the Framingham Heart Study in 1948,1 followed by the advent of multiple other large prospective cohorts (e.g. ARIC, CARDIA, MESA, PESA, UK Biobank), these studies have generated landmark, practice-changing results.2 To date, similar efforts within the field of sports cardiology have been lacking. Accordingly, our contemporary understanding of the natural history and corollary outcomes among competitive athletes with CVD remains rudimentary.
Contemporary Management of Athletes with Cardiovascular Conditions
Sudden cardiac arrest (SCA) attributable to genetic or congenital heart disease remains the leading medical cause of sudden death in young competitive athletes and the leading cause of fatalities during sports and exercise.3 The first expert consensus guidelines in sports cardiology were created at the Bethesda Conference in 19854 after early recognition of the associations between SCA in young athletes and several distinct forms of CVD reported in seminal autopsy studies. This document was written to provide recommendations regarding sports eligibility and disqualification among young competitive athletes diagnosed with CVD. Early Bethesda Conference recommendations, driven largely by expert consensus and opinion, endorsed a strict binary "yes" or "no" clinical decision-making strategy. The last several decades have provided numerous scientific studies and mounting global clinical experience caring for athletes with CVD leading to three important observations. First, the prevalence of most "high-risk" cardiac conditions far exceed contemporary incidence estimates of SCA among young athletes. Second, most of the key diseases associated with SCA exist along a phenotypic spectrum ranging from mild, at times even uncertain "gray zone" expression, to overt severe pathology. Third, disqualification of young competitive athletes has important implications for long-term health and wellness.
The most recent recommendations emerging from the American Heart Association/American College of Cardiology (AHA/ACC) in 2015,5 and European Society of Cardiology (ESC) in 20206 endorse individualized, patient-centered flexibility in the determination of sports eligibility among athletes with CVD. While many practitioners in the sports cardiology and sports medicine communities view these recent updates as progressive and appropriate, it must be acknowledged that the current recommendations remain predominantly based on expert opinion in the absence of definitive long-term outcomes data among athletes with CVD.
The Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) Study
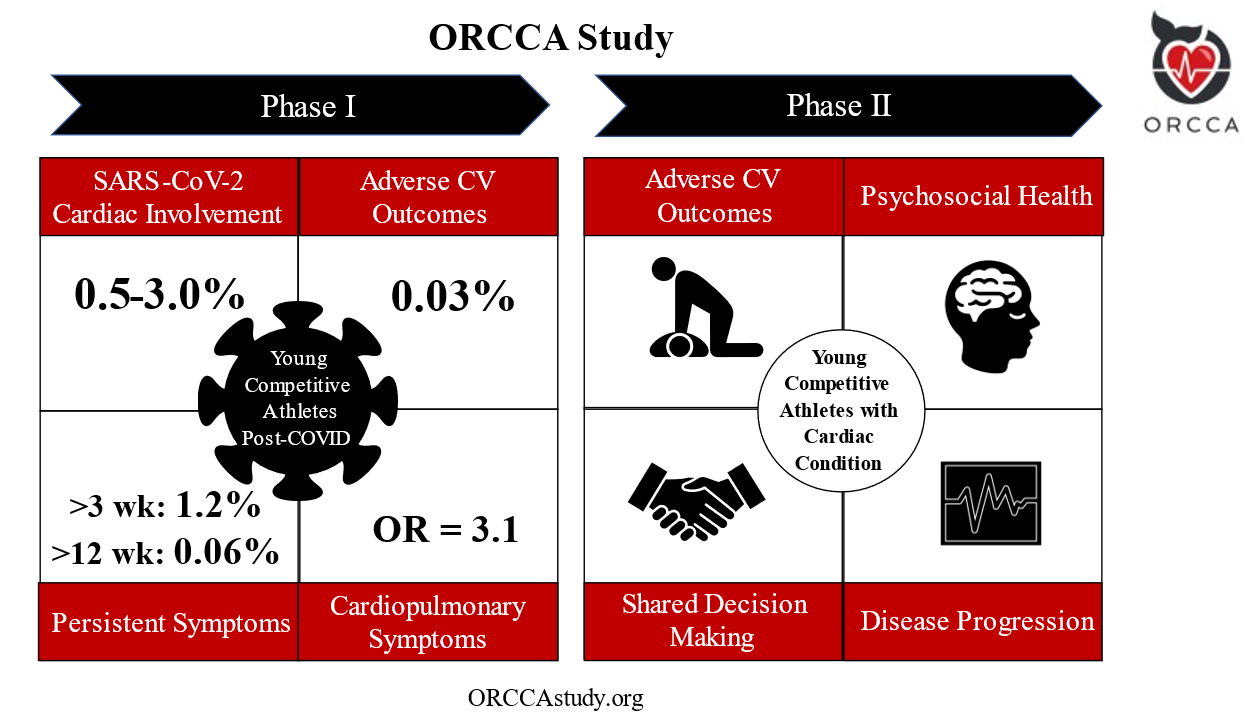
The Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) study was designed to examine clinical outcomes among young competitive athletes with underlying CVD. Established in May of 2020, ORCCA "Phase 1" was dedicated to the study of acquired myocardial and pericardial pathology following SARS-CoV-2 infection. The primary aim of Phase 2, launched in April 2022, is to assess a wide array of longitudinal outcomes among athletes with the key genetic, congenital, and acquired forms of CVD that have been previously associated with SCA. An overview of ORCCA Phase 1 and Phase 2 is presented in Figure 1.
Figure 1
Definition of abbreviations: OR= odds ratio
For Phase 1, the prevalence of SARS-CoV-2 cardiac involvement was found to be 0.5-3.0%.7 On a median follow-up of >1 year (n=3,675), there was one adverse event (0.03%) possibly related to SARS-CoV-2 infection.9 The prevalence of persistent symptoms following SARS-CoV-2 infection >3 weeks was found to be 1.2%, and >12 weeks in 0.06% of athletes.8 Cardiopulmonary symptoms were associated with SARS-CoV-2 cardiac involvement in multivariate analysis with odds ratio 3.1 [95% CI 1.2-7.7].7
Phase 1
The initial phase of ORCCA enrolled 3,685 athletes from 45 colleges/universities following a confirmed diagnosis of SARS-CoV-2 infection. ORCCA Phase 1 resulted in several practice-changing results which include: 1) a low prevalence of SARS-CoV-2 cardiac involvement among athletes following infection (0.5-3%),7 2) the importance of specific cardiopulmonary symptoms, most specifically chest pain during the acute infection or subsequent "return-to-play", as a determinant of the likelihood of SARS-CoV-2 cardiac involvement,7,8 3) a low prevalence of persistent symptoms in athletes >12 weeks (0.06%) despite the high prevalence post-acute sequelae of SARS-CoV-2 (PASC) reported in the general population, and 4) the determination of a reassuringly low prevalence of adverse cardiovascular events related to SARS-CoV-2 infection in >1 year of clinical surveillance following infection (0.03%) (Figure 1).9 Another study also demonstrated that "abnormal" electrocardiographic (ECG) patterns can be found among athletes without evidence of SARS-CoV-2 cardiac involvement on cardiovascular imaging (transthoracic echocardiography or cardiac magnetic resonance imaging).10 The results from ORCCA Phase 1 subsequently informed the most contemporary "return-to-play" recommendations outlined in the 2022 ACC Expert Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults.11
Phase 2
Leveraging the infrastructure and scientific productivity of ORCCA Phase 1, Phase 2 will investigate long-standing, pre-COVID-19 pandemic areas of clinical uncertainty pertaining to clinical management and subsequent outcomes among young competitive athletes with a suspected or confirmed "high-risk" cardiac diagnosis. ORCCA Phase 2 will utilize an online enrollment portal (ORCCAstudy.org) and will seek to enroll competitive athletes aged 18-30 who have been diagnosed with a suspected or confirmed high-risk cardiac diagnosis within the past 2 years (Table 1). Both athletes that stop competitive sports and those that continue are included in this study. Potential participants will either self-identify or be referred by one of their care team members (e.g., cardiologist, sports medicine physician, athletic trainer) through the study website. If a participant meets inclusion criteria after review by the central study team and consents to participate, they will be evaluated at study enrollment and followed every 6 months for longitudinal outcomes data. Athletes will complete REDCap questionnaires for entry into a central data repository housed at the University of Washington (Seattle, WA, USA) at each interval follow-up period, and medical records and cardiovascular testing results will be requested from their care providers.
Table 1: Inclusion Criteria for ORCCA
- Competitive athletes ages 18-30 years old* diagnosed within the past 2 years with one of the following:
- Pathologic Cardiac Condition
- Cardiomyopathy
- Primary Electrical Disease
- Myocarditis
- Coronary Artery Disease/Anomaly
- Congenital Heart Disease
- Valvular Heart Disease**
- Aortopathy
- Cardiac Finding of Unknown Significance
- Markedly Abnormal ECG per the International Criteria12 with Normal Cardiac Imaging^
- Marked Left Ventricular Hypertrophy (≥14mm M, ≥13mm F)
- Aortic Dilatation (≥40mm M, ≥34mm F)
- Subclinical ventricular scar or late-gadolinium enhancement on CMR^^
- Non-compacted LV myocardium with concerns for underlying cardiomyopathy
- Genotype positive/phenotype negative for known pathologic variant of genetic cardiomyopathy or channelopathy
- Pathologic Cardiac Condition
Definition of Abbreviations: CMR= cardiac magnetic resonance imaging, ECG= electrocardiogram, F= female, LV= left ventricular, M= male
*Competitive athlete defined as any individual who places a high premium on exercise training, competition, and sports achievement
**Moderate or greater valvular regurgitation/stenosis, or primary structural abnormality (bicuspid, prolapse, myxomatous, congenital or rheumatic)
^Normal echocardiogram or CMR
^^Excluding isolated right ventricular insertion point late gadolinium enhancement (LGE)
The primary outcome of ORCCA Phase 2 is to determine the prevalence of adverse cardiovascular outcomes, as defined as a composite of major adverse cardiovascular events (MACE) inclusive of SCA. Secondary outcomes include assessment of sports participation status following diagnosis, characterization of the decision-making process between athletes and relevant stakeholders to determine sports eligibility, CVD progression, continued sports and exercise habits, psychological well-being and the development of mental health disorders, and overall quality of life.
Contributing to ORCCA
Individual providers or provider networks who care for competitive athletes with suspected or established CVDs are encouraged to contact the ORCCA investigators to become official collaborators/collaborating sites. All athletes and providers who are interested in participating in ORCCA can find additional information and registration materials at ORCCAstudy.org.
References
- Andersson C, Nayor M, Tsao CW, Levy D, Vasan RS. Framingham Heart Study: JACC Focus Seminar, 1/8. J Am Coll Cardiol 2021;77:2680-92.
- Turco JV, Mearns BM, Fuster V. JACC pivots to history to inspire creativity. J Am Coll Cardiol 2021;77:2747-48.
- Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association athletes: a decade in review. Circulation 2015;132:10-19.
- Mitchell JH, Maron BJ, Epstein SE. 16th Bethesda Conference: cardiovascular abnormalities in the athlete: recommendations regarding eligibility for competition. J Am Coll Cardiol 1985;6:1186-1232.
- Maron BJ, Zipes DP, Kovacs RJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: preamble, principles, and general considerations. J Am Coll Cardiol 2015;66:2343-49.
- Pelliccia A, Sharma S, Gati S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: the task force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur Heart J 2020;42:17-96.
- Moulson N, Petek BJ, Drezner JA, et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation 2021;144:256-66.
- Petek BJ, Moulson N, Baggish AL, et al. Prevalence and clinical implications of persistent or exertional cardiopulmonary symptoms following SARS-CoV-2 infection in 3597 collegiate athletes: a study from the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA). Br J Sports Med 2021;Nov 01;[Epub ahead of print]..
- Petek BJ, Moulson N, Drezner JA, et al. Cardiovascular outcomes in collegiate athletes following SARS-CoV-2 infection: 1-year follow-up from the outcomes registry for cardiac conditions in athletes. Circulation 2022;May 12:[Epub ahead of print].
- Petek BJ, Moulson N, Baggish AL, et al. Electrocardiographic findings in young competitive athletes during acute SARS-CoV-2 infection. J Electrocardiol 2022;72:13-15.
- Gluckman TJ, Bhave NM, Allen LA, et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play. J Am Coll Cardiol 2022;79:1717-56.
- Drezner JA, Sharma S, Baggish A, et al. International criteria for electrocardiographic interpretation in athletes: consensus statement. Br J Sports Med 2017;51:704-31.
Clinical Topics: Arrhythmias and Clinical EP, Congenital Heart Disease and Pediatric Cardiology, COVID-19 Hub, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Sports and Exercise Cardiology, Valvular Heart Disease, Atherosclerotic Disease (CAD/PAD), Genetic Arrhythmic Conditions, Congenital Heart Disease, CHD and Pediatrics and Arrhythmias, CHD and Pediatrics and Imaging, CHD and Pediatrics and Quality Improvement, Echocardiography/Ultrasound, Magnetic Resonance Imaging, Sports and Exercise and Congenital Heart Disease and Pediatric Cardiology, Sports and Exercise and Imaging
Keywords: Sports, Sports Medicine, Athletes, COVID-19, SARS-CoV-2, Contrast Media, Gadolinium, Cardiovascular Diseases, American Heart Association, Biological Specimen Banks, Channelopathies, Channelopathies, Cicatrix, Clinical Decision-Making, Clinical Decision-Making, Coronary Artery Disease, Dilatation, Expert Testimony, Follow-Up Studies, Hypertrophy, Left Ventricular, Myocarditis, Pandemics, Prospective Studies, Return to Sport, Electrocardiography, Myocardium, Myocardium, Echocardiography, Magnetic Resonance Imaging, Heart Defects, Congenital, Heart Valve Diseases, Heart Valve Diseases, Longitudinal Studies, Longitudinal Studies, Multivariate Analysis, Patient-Centered Care, Medical Records, Risk Factors, Patient Care Team, Chest Pain, Registries, Genotype
< Back to Listings