5-FU-Associated Coronary Vasospasm: Who is Really at Risk?
Quick Takes
- In the largest single-center retrospective cohort of 5-fluorouracil (5-FU) associated coronary vasospasm, younger patients with lower rates of baseline cardiovascular risk factors were at a higher risk of developing coronary vasospasm.
- The 5-FU dose received by patients with vasospasm was significantly lower than that for patients without vasospasm; however, there was no significant difference in progression-free or overall survival among patients with coronary vasospasm compared with those who did not develop vasospasm.
- Younger patients may require closer monitoring and lower threshold for workup of any chest discomfort in the setting of 5-FU therapy.
Background
5-FU is the third most common drug used for the treatment of solid malignancies, including adenocarcinomas of the breast, head, and neck and gastrointestinal tract and bladder cancer.1,2 However, it is the second most common drug associated with cardiotoxicity after anthracyclines.1 The most common reported symptom is chest pain thought to be secondary to vasospasm.1,3 The reported incidence of 5-FU-associated cardiotoxicity varies from 1% to 35% due to differences in the sample population, definitions of 5-FU-associated cardiotoxicity, and the varied formulations/administration protocols of 5-FU.4 The goal of this study was to assess the incidence, risk factors, and prognostic implications of 5-FU-associated coronary vasospasm among patients receiving 5-FU therapy at a single center.
Methods
The study conducted a retrospective analysis of all patients who received 5-FU at a single academic center from January 2009 to July 2019. Vasospasm was defined as the occurrence of a typical chest pain syndrome in the setting of 5-FU therapy, associated with either electrocardiographic changes or elevated cardiac biomarkers. Individuals were initially flagged on keyword search for 5-FU and vasospasm and recent diagnosis of myocardial infarction. The charts were then manually reviewed, and the diagnosis of vasospasm was independently adjudicated by two cardiologists. Patients with vasospasm were compared to patients without vasospasm in a 1:2 fashion. Kaplan-Meier methods and log-rank test were used to compare median overall survival and progression-free survival. Furthermore, Cox proportional hazards regression was used to calculate hazard ratios for overall mortality, cancer-related mortality, and progression of underlying cancer.
Results
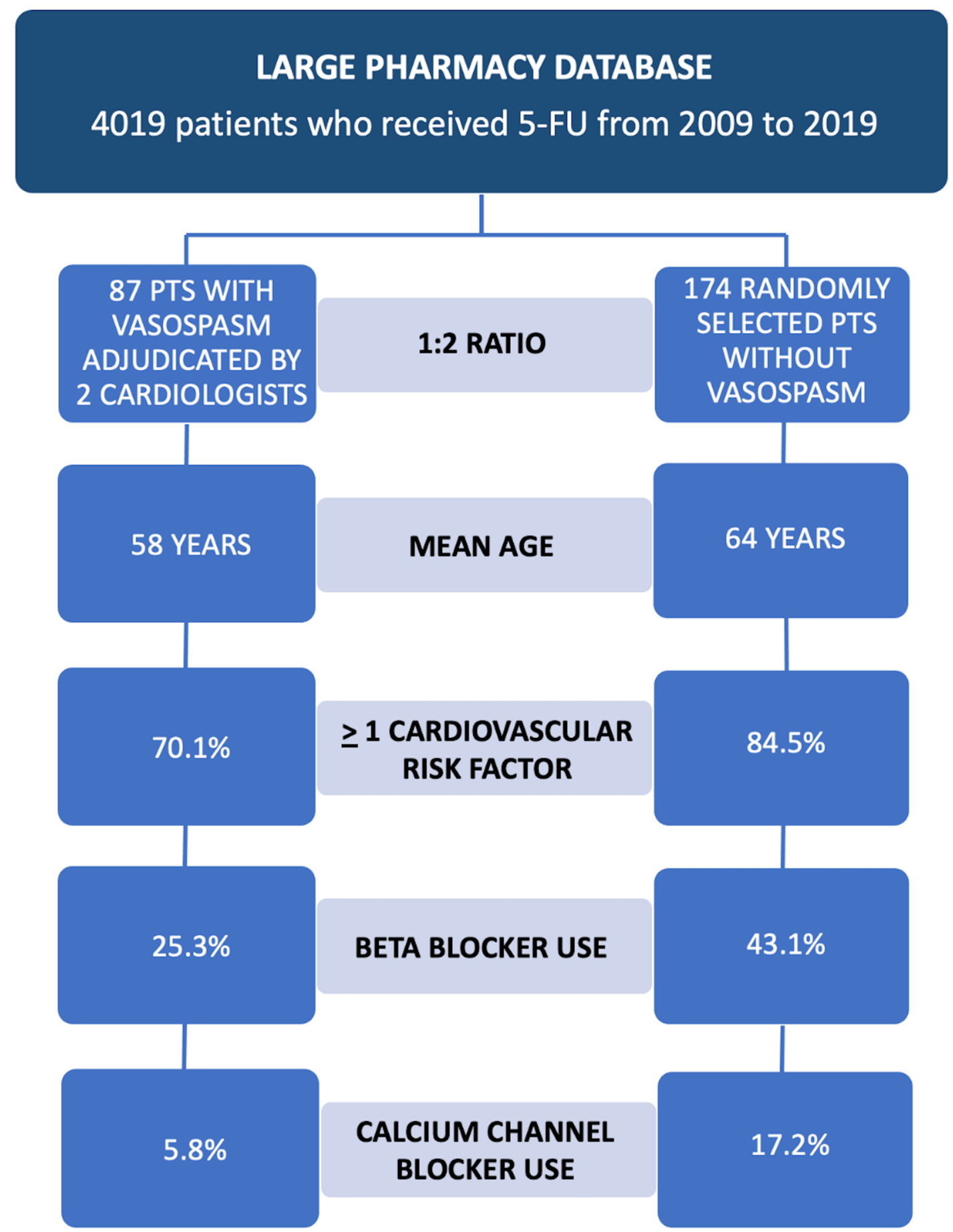
Over 4,000 patients received 5-FU therapy over a period of 10 years at a single center (Figure 1). Of those patients, 87 (2.16%) developed vasospasm as defined by study criteria. Out of the 3,932 patients who received 5-FU but did not develop vasospasm, 174 were randomly selected for comparison. Patients who developed vasospasm were younger (age 58 ± 13 years vs. 64 ± 13 years; p = 0.001). The 2 groups were also similar in terms of type and stage of cancer. Patients with vasospasm were less likely to have any cardiovascular risk factors (70.1% with at least 1 cardiovascular risk factor for patients with vasospasm vs. 84.5% for patients without vasospasm; p = 0.007). Patients with vasospasm were less likely to be on any cardiac medications, including aspirin (31.0% vs. 42.0%; p = 0.087), beta-blockers (25.3% vs. 43.1%; p = 0.005), and calcium channel blockers (5.8% vs. 17.2%; p = 0.01). The total median 5-FU dose adjusted for body surface area received by patients with vasospasm was lower than that for patients without vasospasm (5,388 [interquartile range: 2,800-10,310] mg/m2 vs. 11,241 [interquartile range: 7,710-24,288] mg/m2; p < 0.001). The median progression-free survival for patients with vasospasm was 553 days (95% confidence interval [CI]: 427-924) versus 608 days (95% CI: 456-833) for patients without vasospasm (p = 0.77). The median overall survival for patients with vasospasm was 1,277 days (95% CI: 780-2,039) versus 1,150 days (95% CI: 822-1,637) for patients without vasospasm (p = 0.57).
Figure 1: Study Design and Key Differences Between Patients Who Developed 5-FU-Associated Coronary Vasospasm and Those Who Did Not
Conclusions
In the largest, single-center report of 5-FU-associated vasospasm, patients who developed vasospasm were younger, had lower rates of traditional cardiovascular risk factors, and received lower cumulative doses of 5-FU.
Perspective
We hypothesize that the lower prevalence of coronary vasospasm among patients with known cardiovascular risk factors is due to the concurrent use of cardioprotective medications such as aspirin, beta-blockers, and calcium channel blockers. However, further research is required to test this hypothesis and study whether the prophylactic use of these medications prior to rechallenging patients with known 5-FU-associated vasospasm is both safe and efficacious.
References
- Sara JD, Kaur J, Khodadadi R, et al. 5-fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol 2018;10:1758835918780140.
- Layoun ME, Wickramasinghe CD, Peralta MV, Yang EH. Fluoropyrimidine-Induced Cardiotoxicity: Manifestations, Mechanisms, and Management. Curr Oncol Rep 2016;18:35.
- Depetris I, Marino D, Bonzano A, et al. Fluoropyrimidine-induced cardiotoxicity. Crit Rev Oncol Hematol 2018;124:1-10.
- Zafar A, Drobni ZD, Mosarla R, et al. The Incidence, Risk Factors, and Outcomes With 5-Fluorouracil-Associated Coronary Vasospasm. JACC CardioOncol 2021;3:101-9.
Clinical Topics: Cardio-Oncology, Stable Ischemic Heart Disease, Statins, Chronic Angina
Keywords: Calcium Channel Blockers, Cardiotoxicity, Fluorouracil, Coronary Vasospasm, Anthracyclines, Prognosis, Disease-Free Survival, Retrospective Studies, Urinary Bladder Neoplasms, Confidence Intervals, Aspirin, Pharmaceutical Preparations, Cardiovascular Diseases, Risk Factors, Chest Pain, Myocardial Infarction, Adenocarcinoma, Gastrointestinal Tract, Biomarkers, Breast Neoplasms
< Back to Listings