Feature Story: EuroPCR 2017 and SCAI 2017; Some Highlights
The evidence base for transcatheter aortic valve replacement (TAVR) in patients with severe aortic stenosis (AS) continues to grow and contributions from EuroPCR 2017 and the Society for Cardiovascular Angiography and Interventions (SCAI) 2017 Scientific Sessions provide more insights into the impact of atrial fibrillation (AFib), the type of valve, and embolic protection, as well as data from the first head-to-head valve comparison.
New data from the BRAVO-3 trial presented at SCAI showed that more than a third of patients undergoing TAVR had AFib either at baseline or new-onset AFib within 30 days after TAVR. The risk of stroke within 30 days was more than four-fold greater in the patients with new onset-AFib.
"Previous studies have shown that AFib patients undergoing TAVR have a greater risk for ischemic as well as bleeding events," said the study’s principal investigator George D. Dangas, MD, PhD, FACC. "We wanted to study these associations using a more contemporary population, in the context of overall improved devices, techniques and post-TAVR management to see if the results were similar. We were also interested in studying the antithrombotic prescription patterns in this high-risk patient population, as there is currently no consensus on the optimal strategy following TAVR."
" It is not the differences in the valves, but differences in the patients. When we select the valve based on the patient’s characteristics, the outcomes are positive." — Roxana Mehran, MD, FACC
BRAVO-3 included 802 patients with severe AS and a mean age of 82 years randomized to bivalirudin or unfractionated heparin for transfemoral TAVR. For the primary endpoint of 48-hour Bleeding Academic Research Consortium (BARC) type 3b bleeding in the main study, no advantage was seen with bivalirudin vs. heparin for bleeding outcomes or net adverse clinical events (death, myocardial infarction [MI], stroke and major bleeding) up to 30 days.
In this sub-study, 41.4 percent (n = 332) of patients had baseline AFib or new-onset AFib within 30 days of TAVR, and 58.6 percent (n = 470) had no AFib. Patients with existing or new-onset AFib had a greater occurrence of pre-existing comorbidities, including renal dysfunction, lower left ventricular ejection fraction (LVEF) and a higher EuroSCORE I.
Unadjusted 30-day clinical outcomes for AFib vs. non-AFib patients were stroke (3.9 vs. 2.6 percent), death (6.0 vs. 4.5 percent) and BARC major bleeding (10.2 vs. 9.4 percent). The unadjusted odds of early stroke were 4.49-fold greater with new-onset AFib vs. no AFib (95 percent confidence interval [CI], 1.37-14.67). No significant differences were noted in 30-day outcomes by AFib status and anticoagulant strategy.
Most AFib patients had a CHA2DS2VASc score of 4 (10 percent) or higher (35 percent had a score of 5 and 31 percent a score of 6). At discharge, 30 percent were sent home with dual antiplatelet therapy (DAPT) and no anticoagulant, 32 percent with aspirin and an oral anticoagulant, 22 percent with a P2Y12 inhibitor and an oral anticoagulant and 13 percent on triple therapy. Eighty percent of patients without AFib were given DAPT at discharge.
"Although nearly all AFib patients had a high calculated predictive stroke risk score (CHA2DS2VASc) of ≥4, we found that around two-thirds went home on an oral anticoagulant. We need to better risk stratify AFib patients during follow-up, when acute risk of bleeding has abated, for prescription of anticoagulants where appropriate," said Usman Baber, MD, who presented the study.
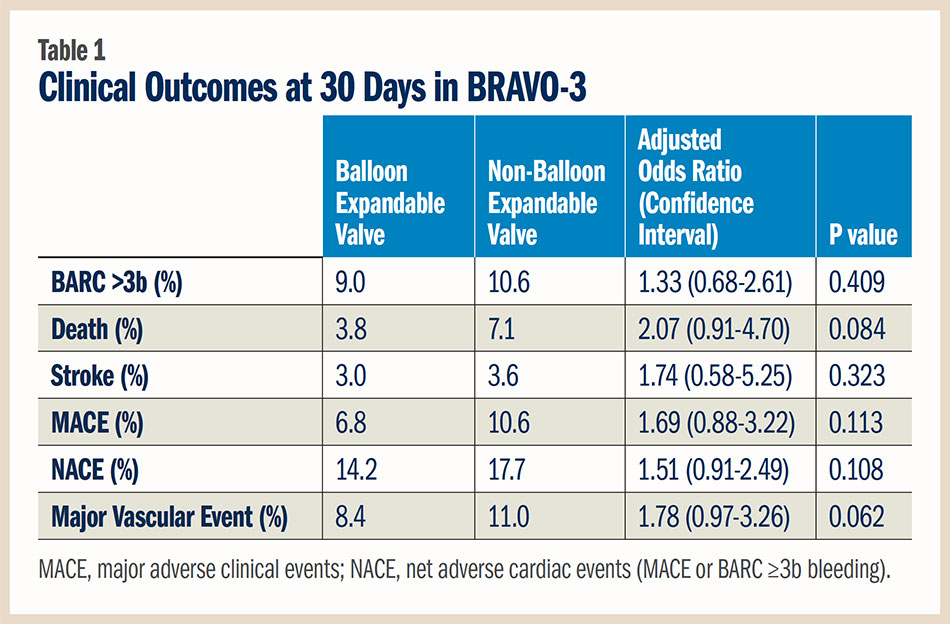
Also presented at SCAI, a pre-specified subgroup analysis from BRAVO-3 found that patient risk factors — not the type of valve — determined 30-day outcomes post TAVR. Balloon expandable valves were implanted in 500 patients and non-balloon expandable in 282 patients. Although selection of valve type was at the operator’s discretion, randomization to bivalirudin or unfractionated heparin was stratified by valve type.
The patients receiving balloon expandable valves had a different baseline profile than patients treated with non-balloon expandable valves, who were older and had a higher EuroSCORE I but lower rates of diabetes. They also had a trend for higher prevalence of chronic obstructive lung disease, lower LVEF and lower body weight. Within the balloon expandable vs. non-balloon expandable valve groups, 251 vs. 140 were treated with bivalirudin and 249 vs. 142 with unfractionated heparin.
Although patients who received a non-balloon expandable valve were more likely to require a second implant during the procedure, there were no significant differences in the adjusted endpoints between the groups. "This is not a one size fits all situation," said Roxana Mehran, MD, FACC. "It is not the differences in the valves, but differences in the patients. When we select the valve based on the patient’s characteristics, the outcomes are positive." She also cautioned that the study was not powered to evaluate outcomes by valve type. Further, the studies are not generalizable to other TAVR studies that used both transfemoral and non-transfemoral approaches.
The 30-day clinical outcomes are summarized in Table 1. There was a trend for a higher adjusted risk of major vascular complications with non-balloon expandable valves, which may be a function of available device generation during the study period. There was a borderline interaction between valve type and anticoagulation treatment for the occurrence of major vascular complications, suggesting that bivalirudin might be associated with lower risk in patients treated with non-balloon expandable valves. However, Mehran cautioned that these are small numbers of patients.
Mehran also noted that the new requirement for permanent pacemaker and residual aortic incompetence post TAVR was not recorded, yet the study provides relevant comparative data on outcomes by valve type in contemporary TAVR.
"What is unique about our study is that, counter to prevailing assumptions, it shows that you can rapidly enroll women and minorities into a prospective registry and collect statistically valid data." — Wayne Batchelor, MD, MHS, FACC
The international REPRISE III study, presented by Ted Feldman, MD, FACC, at EuroPCR, the first head-to-head comparison of TAVR valves, found that the Lotus valve system was non-inferior to the CoreValve first-generation and Evolut R valves for the 30-day primary safety endpoint and superior for the one-year primary effectiveness endpoint in patients with severe AS at high or extreme surgical risk.
The Lotus valve system does not have U.S. Food and Drug Administration approval, but has CE mark approval for use in Europe. In February 2017, Boston Scientific recalled all Lotus valves worldwide because of issues related to the locking mechanism.
In REPRISE III, 912 patients were randomized 2:1 to the Lotus valve or CoreValve (about half to the CoreValve Evolut R) at centers in the U.S., Germany, France and Australia.
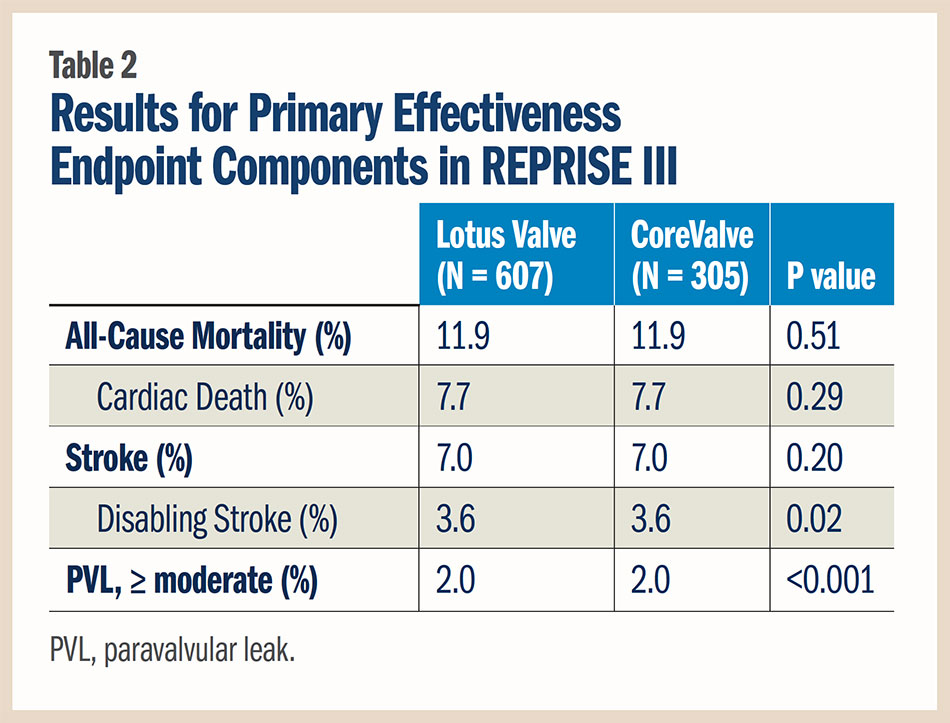
The 30-day primary composite safety endpoint, consisting of all-cause mortality, stroke, life-threatening/major bleeding, stage 2/3 acute kidney injury and major vascular complications, occurred in 19.0 percent of patients in the Lotus valve group and 16.2 percent in the CoreValve group in the intent-to-treat analysis (p for non-inferiority 0.001). A similar outcome was seen when analyzed by patients receiving the assigned valve, at 20.3 percent with the Lotus valve and 17.2 percent with the CoreValve (p for non-inferiority 0.003). Further, at one year, no difference was seen between valves for this endpoint (30.7 vs. 30.2 percent, respectively; p = 0.83).
The rate of new pacemaker implantation at 30 days was nearly two-fold higher in patients treated with the Lotus valve than with the CoreValve (29.1 vs. 15.8 percent; p < 0.001). The need for aortic valve repositioning was greater in the CoreValve group (2.6 percent vs. 0 in the Lotus group; p < 0.001).
For the primary effectiveness endpoint, consisting of death, disabling stroke and moderate or greater paravalvular leak at one year, the Lotus device was also found to be non-inferior to the CoreValve in the intent-to-treat analysis (16.7 vs. 29.0 percent, respectively; p for non-inferiority < 0.001) and in the as-treated analysis (16.4 vs. 28.6 percent, respectively; p for non-inferiority < 0.001). The Lotus valve also met criteria for superiority vs. the CoreValve for the primary effectiveness endpoint. The results for the components of the primary effectiveness endpoint are detailed in Table 2.
In the arena of embolic protection during TAVR, a meta-analysis presented at EuroPCR and simultaneously published in Stroke showed that it may be associated with a smaller volume of silent ischemic lesions and smaller total volume of silent ischemic lesions — but it may not reduce the number of new single or multiple lesions or the total number of lesions. Regarding clinically evident stroke and 30-day mortality, no significant difference was seen between the patients who did and did not have embolic protection during TAVR, but the authors caution that there was only very quality evidence for this finding.
The meta-analysis included 1,170 patients, of whom 865 had embolic protection, in 16 studies. Delivery of the embolic protection device was achieved in 94.5 percent of patients. The relative risk for clinically evident stroke was 0.70 (95 percent CI, 0.38-1.29; p = 0.26) and for 30-day mortality it was 0.58 (95 percent CI, 0.20-1.64; p = 0.30). The standardized mean difference in ischemic volume per lesion was —0.52 (p = 0.002) and smaller total volume of lesions was —0.23 (p = 0.02).
Noting that the concern about the potential impact of silent ischemic lesions on neurocognitive function will be extended to younger and less sick patients as TAVR continues to move towards treating these patients, Rodrigo Bagur, MD, PhD, and colleagues state that more investigation is needed to better identify the patients at high risk for embolization.
Data from the SURTAVI trial presented by A. Pieter Kappetein, MD, PhD, at EuroPCR 2017 showed that the early rate of 30-day stroke was significantly lower with TAVR than with surgical aortic valve replacement (3.3 vs. 5.4 percent, respectively; log-rank p = 0.031). At two years, the rates were 6.3 vs. 8.0 percent (log-rank p = 0.143). Neurological events were adjudicated by an independent committee and the Valve Academic Research Consortium-2 definition of stroke was used.
Baseline characteristics were similar regardless of stroke status. Quality of life improved faster for TAVR patients who had a stroke and at 30 days it was significantly better than for SAVR patients, although it was similar at six months.
Diversity Matters in Clinical Trials, Outcomes Differ in PCI
Race, gender and socioeconomic factors were shown to impact PCI outcomes in what was called a first-of-its-kind study presented at SCAI 2017. The sub-analysis of the PLATINUM Diversity study, the first prospective study to enroll only women and minorities, found they had a greater risk of recurrent cardiac events within the first year after PCI compared with Caucasian men.
"We wanted to design a study to prospectively examine outcomes across a large sample of women and minorities and compare that with the same stent used in recent studies of large numbers of Caucasian men," said Wayne Batchelor, MD, MHS, FACC, co-principal investigator along with Mehran. He added, "What is unique about our study is that, counter to prevailing assumptions, it shows that you can rapidly enroll women and minorities into a prospective registry and collect statistically valid data."
PLATINUM Diversity enrolled U.S. patients who received one Promus PREMIER stent and self-identified as being female, black (of African heritage), Hispanic/Latino or American Indian/Alaskan Native. To enrich the study with women and minorities and allow for comparisons with white men, PLATINUM Diversity patients (n = 1,501) were pooled with the Promus-Element Plus Post Approval Study patients (n = 2,687) for a total study sample of 4,188 patients.
Within this large cohort, there were 1,417 white and 427 minority women and 1,635 white and 632 minority men available for analysis. The subgroup analysis evaluated one-year outcomes in both minority and white men and women and the inter-related effects of sex and race.
For the primary endpoint of death, MI and target vessel revascularization at 12 months, minority women had a 50 percent increased adjusted risk compared with white men. The rates of the primary endpoint were 11.7 percent for minority women, 8.4 percent for minority men, 8.1 percent for white women and 7.9 percent for white men. Minority women had a nearly three-fold increased risk of death and MI that appeared to be mainly driven by a nearly three-fold increase in the likelihood of an MI within a year of receiving a contemporary drug-eluting stent.
"What we found was that there were significant differences in adjusted outcomes between these groups, with especially higher risks of cardiac events in minority women at one year," Batchelor stated. "However, these incremental risks appeared to be related to progression of the patient’s ischemic heart disease more than failure of the stent due to stent thrombosis or restenosis."
Disrupting CAD
A new technology designed to treat calcified coronary artery blockages prior to drug-eluting stenting with lithotripsy, sonic pressure waves historically used to treat patients with kidney stones, was shown in the DISRUPT CAD study to provide a high acute gain with low residual stenosis. The six-month results were presented by Todd J. Brinton, MD, FACC, at EuroPCR.
Later in May, CE mark approval was gained for the Coronary Lithoplasty® System for the treatment of calcified plaque in conjunction with stenting in patients with coronary artery disease (CAD).
In the single arm study, Brinton and colleagues enrolled 60 patients with moderately and severely calcified de novo coronary lesions with a reference vessel diameter between 2.5 and 4.0 mm, at least 50 percent stenosis and a length ≤32 mm. Patients had evidence of ischemia, symptoms or both. Mean lithoplasty inflation pressure was 6 atm and most patients received post-dilatation.
All but one patient had successful treatment with the device and all had successful stent delivery. The core lab evaluation showed the total acute gain was 1.7 mm. The primary endpoint of major adverse clinical events at six months was 8.5 percent, including two non-procedure related cardiac deaths at six months.
What’s next for this technology? It’s now being investigated for peripheral arterial disease and for transcatheter treatment of aortic valve stenosis.
Tweet this article: Tweet
 |
|
| Click the cover image above to read the latest issue of Cardiology Interventions in e-pub format or click here to read it on the web! | |
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Cardiac Surgery, Cardiovascular Care Team, Invasive Cardiovascular Angiography and Intervention, Valvular Heart Disease, Anticoagulation Management and Atrial Fibrillation, Atrial Fibrillation/Supraventricular Arrhythmias, Aortic Surgery, Cardiac Surgery and Arrhythmias, Cardiac Surgery and VHD, Interventions and Imaging, Interventions and Structural Heart Disease, Angiography, Nuclear Imaging
Keywords: ACC Publications, Cardiology Interventions, Heparin, Aortic Valve Insufficiency, Anticoagulants, Risk Factors, Transcatheter Aortic Valve Replacement, Atrial Fibrillation, Comorbidity, Consensus, Prospective Studies, Stroke Volume, United States Food and Drug Administration, Aortic Valve Stenosis, Stroke, Myocardial Infarction, Diabetes Mellitus, Pulmonary Disease, Chronic Obstructive, Angiography
< Back to Listings


