A 32-year-old male competitive swimmer with probable familial hyperlipidemia (with a Dutch Lipid Clinic Network score [DLCNS] of 6 points) presents with exertional chest pain. His family history is notable for sudden, unexplained death in his 38-year-old brother, presumed to be cardiac in nature. The family decided to pursue neither autopsy nor genetic testing. He is an elite sprint swimmer training for Olympic trials and describes brief episodes of exertional chest discomfort at peak exertion with swimming. The chest pain subsides with rest, and he cannot reproduce the chest pain with other physical activity, including dry-land training. In total, he trains approximately 30 hours per week, with 20 hours dedicated to swimming and 10 hours of dry weight lifting and light aerobic activity. He states that he has had elevated lipid levels for several years; recently, his low-density lipoprotein cholesterol (LDL-C) level was 218 mg/dL. His only medication is rosuvastatin 5 mg, which was started a few weeks earlier; he reports some muscle cramping since starting this medication. He does not endorse any previous or current smoking, alcohol, or illicit drug or supplement use.
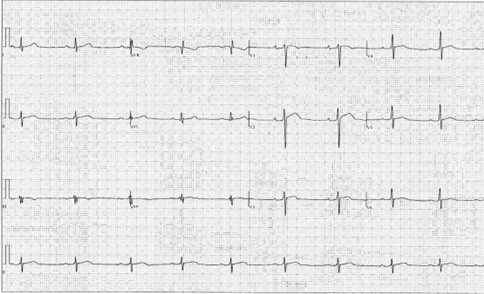
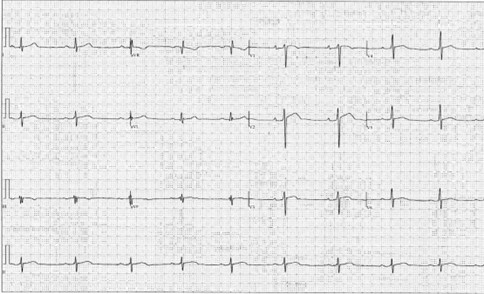
His blood pressure (BP) is 140/85 mm Hg and heart rate is 54 bpm. His cardiac examination findings are unremarkable. He does not have any appreciable tendon xanthomas or xanthelasmas. An electrocardiogram (ECG) is performed (Figure 1). A transthoracic echocardiogram shows normal chamber sizes, normal biventricular function except for a reduced left ventricular (LV) global longitudinal strain (GLS) of -13.8%, normal diastolic function, and no significant valvular abnormalities.
Figure 1
Given his risk factors, including a family history of presumed sudden cardiac death (SCD) in his older brother, and exertional symptoms, the decision is made to pursue a coronary computed tomography angiogram (CCTA) to define his coronary anatomy and evaluate for any potential atherosclerotic burden.
He asks whether he can, in the interim, continue his high level of swimming and training to pursue his goal of qualifying for the Olympics. Both he and the clinician are aware that detraining over a few weeks while waiting for evaluation to conclude will be detrimental to his Olympic pursuit.
The correct answer is: C. There is a nonzero risk of cardiac events with continued training and his cardiac evaluation findings will help further risk stratify him, but the decision to continue training or not in the interval is his to make.
In sports cardiology, there are two key "known unknowns." First, the absolute risk of continued competitive sport participation is incompletely understood. Second, the benefits of sports participation are difficult to quantify but cannot be ignored. Although the findings of early case series have associated the risk of death with activity, the absolute risk per each individual bout of acute exercise is difficult to ascertain, and subsequent studies have demonstrated similar or greater risk of death with nonsport activities. Furthermore, phenotypic variability within conditions makes risk assessment even more difficult. The psychological impact of restriction, even in the short term, can lead to depression, disconnection with medical providers and clinicians, and continued exercise in unsupervised settings. Therefore, a shared decision-making (SDM) model regarding participation in high-level athletics is essential.1 The SDM model between a sports cardiologist and an athlete encompasses five key tenants: knowledge, humility, respect, teamwork, and communication.2
SDM hinges on the principle of reducing information asymmetry, a situation in which one party possesses more in-depth and higher-quality information than the other. Although this concept is often discussed in economic transactions, such as when buying a used car, it is equally if not more vital in health care. In sports cardiology, clinicians bring medical expertise to the consultation room, but patients are the experts in their experiences, preferences, and values. Consequently, the sports cardiologist's initial role is to confirm the correct diagnosis, assess risk, provide education, and treat the athlete. The sports cardiologist must actively minimize information asymmetry by eliciting an athlete's motivations, goals, and purpose. They must explain the implication of their diagnosis on the athlete's sport while recognizing limitations in the understanding of the interaction between certain cardiovascular conditions and exercise. Taken together, the SDM model should seek to eliminate the asymmetry in the perception and interpretation of risk by putting the risk into context of the individual patient-athlete.
With the clinician's knowledge of the disease and the athlete's knowledge of their personal preferences, morals, and values, "symmetric" SDM conversations can occur. These conversations should involve counseling and explaining the potential risks, potential benefits, and likelihood of both. As initially outlined by Levine and Stray-Gundersen in 1994, "the final decision and responsibility rests with the informed patient, who must synthesize this information with assistance from family and friends, in light of his or her own goals and aspirations."3 Nevertheless, it is important to note that the sports cardiologist is not absolved from communicating a recommendation about the advisability of continued competitive athletics.
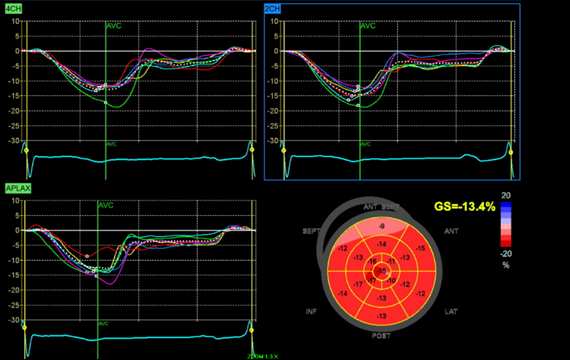
In this 32-year-old swimmer with probable FH as determined by his DLCNS and exertional-like anginal symptoms, his risk of a cardiac event with peak exertion was not negligible, especially given the presumed SCD in his brother and his own reduced LV GLS (Figure 2). GLS has emerged as a useful tool to differentiate pathological LV hypertrophy and maladaptive remodeling from physiological remodeling in athletes. Although a proportion of elite athletes have abnormal GLS, this finding has generally been observed in those with increased LV mass and concentric geometry.4 Although the impact of exercise on GLS is dependent on the specific sport or activity, overall, the evidence suggests that exercise alone should not significantly lower GLS.5 The findings of a study evaluating GLS and myocardial work in human athletes and control patients demonstrated mean GLS 17% in elite male swimmers and 18.2% in elite female swimmers.6 An abnormal GLS in patients in both ischemic and nonischemic cardiomyopathy has been associated with SCD.7 In a postmortem study of presumed SCDs, autopsy-defined sudden arrhythmic death was associated with abnormal GLS among those with available premortem echocardiograms.8
Figure 2: Longitudinal Strain Plot Demonstrating Abnormal Longitudinal Strain With GLS of -13.4%
Figure 2: Longitudinal Strain Plot Demonstrating Abnormal Longitudinal Strain With GLS of -13.4%. Courtesy of Bavishi A, Husaini M.
As demonstrated in this case, sports cardiologists are often required to provide exercise recommendations while a cardiac workup is ongoing. This athlete's discussion included the fact that it would have likely taken a few weeks for the CCTA to be completed and, in that time frame, it would not be unreasonable to either continue training or decrease/withhold training.
Certain aspects of the history and initial workup can influence how reasonable the sports cardiologist believes it is to continue training. In this case, the athlete did not have progression of his symptoms and had mostly reassuring cardiac diagnostic test findings, including physical examination, ECG (Figure 1), and echocardiographic findings (reduced LV GLS notwithstanding). "Red flag" aspects of the history, physical examination, and diagnostic evaluation that would have potentially led to restriction of exercise during the diagnostic workup would include progressing symptoms (duration, frequency, or severity), chest pain or fatigue leading to premature termination of exercise, nonvasovagal syncope or presyncope, markedly abnormal ECG findings, and/or evidence of structural cardiac disease on echocardiography.
Figure 1: Twelve-Lead ECG Demonstrating Sinus Bradycardia, Normal Axis, and First-Degree Atrioventricular Block
Figure 1: Twelve-Lead ECG Demonstrating Sinus Bradycardia, Normal Axis, and First-Degree Atrioventricular Block. Courtesy of Bavishi A, Husaini M.
There were mild early repolarization changes and inferior lead fractionation but no pathological Q waves.
The patient–clinician discussion also included the fact that it was unclear whether his risk of sudden cardiac arrest was higher while swimming than while doing other activities such as strenuous housework or emotional conversations. Paramount to his ability to safely exercise and train in the interim included swimming in a supervised environment with a well-defined and rehearsed emergency action plan. This plan should have included communicating his potential risk to his coaches, athletic trainers, and other relevant personnel, and ensuring the availability of a functioning automated external defibrillator and widespread knowledge of cardiopulmonary resuscitation.
His CCTA demonstrated normal coronary anatomy with a very small, calcified plaque in the mid left anterior descending artery with minimal luminal stenosis. There was no other evidence of plaque or obstructive epicardial stenosis. Given his probable FH and the presence of premature coronary artery calcification, aggressive risk-factor modification was recommended along with maximal-effort cardiopulmonary exercise testing. Follow-up ambulatory BP readings demonstrated systolic BPs in the 110-120s mm Hg. A repeat echocardiogram in a year was scheduled to ensure his abnormal GLS was not an early manifestation of a cardiomyopathy. Aggressive LDL-C level reduction was recommended, with planned follow-up LDL‑C level evaluation to a target goal of ≤55-70 mg/dL. Initiating and maintaining statin therapy in athletes can be challenging. In a study of 22 professional Austrian athletes with FH, only six could tolerate statin treatment because of adverse effects.9 In athletes who are intolerant of statins, strong consideration should be given to alternative agents, such as proprotein convertase subtilisin/kexin type 9 inhibitors, ezetimibe, bempedoic acid, and inclisiran.10
References
- Baggish AL, Ackerman MJ, Putukian M, Lampert R. Shared decision making for athletes with cardiovascular disease: practical considerations. Current Sports Med Rep 2019;18:76-81.
- Isath A, Koziol KJ, Martinez MW, et al. Exercise and cardiovascular health: a state-of-the-art review. Prog Cardiovasc Dis 2023;Apr 28:[ePub ahead of print].
- Levine BD, Stray-Gundersen J. The medical care of competitive athletes: the role of the physician and individual assumption of risk. Med Sci Sports Exerc 1994;26:1190-2.
- Lin J, Wang F, Weiner RB, et al. Blood pressure and LV remodeling among American-style football players. JACC: Cardiovasc Imaging 2016;9:1367-76.
- Murray J, Bennett H, Bezak E, Perry R, Boyle T. The effect of exercise on left ventricular global longitudinal strain. Eur J Appl Physiol 2022;122:1397-408.
- Tokodi M, Oláh A, Fábián A, et al. Novel insights into the athlete's heart: is myocardial work the new champion of systolic function? Eur Heart J Cardiovasc Imaging 2022;23:188-97.
- Buss SJ, Breuninger K, Lehrke S, et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging 2015;16:307-15.
- Ramakrishna S, Bibby D, Vittinghoff E, et al. Abstract 14216: Sudden arrhythmic death is associated with lower global longitudinal strain and longer mechanical dispersion in the San Francisco Postmortem Systematic Investigation of Sudden Cardiac Death (POST SCD) study. Circulation 2019;140:A14216.
- Sinzinger H, O'Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol 2004;57:525-8.
- Bavishi A, Zielinski A, Stone NJ. Risk and treatment decisions for primary prevention in the athlete: worth going the extra mile. Am J Cardiol 2022;179:129-31.