Initial Presentation
| *Chief Complaint: |
Palpitations |
| History of Present Illness: |
A 48-year-old woman presents with increasingly frequent palpitations associated with effort intolerance. She has had episodic spells for several years, including an episode 2 years earlier of acute-onset rapid, irregular palpitations, crushing chest pain, and shortness of breath while at rest. She was found to have atrial fibrillation with sinus rhythm returned within 12 hours. Over the previous year, she has had milder recurrences with increasing frequency. |
History
| Other Medical History (include comorbidities, implants, etc.): |
Chronic watery diarrhea (4 years)
Mild renal impairment, chronic kidney disease, baseline creatinine of 1.7 g/dL |
| Family History: |
Mother with "heart block" requiring a pacemaker; died of heart failure at 89 years of age
Sister with atrial fibrillation, onset in her 50s
Brother with renal failure in his 20s leading to renal transplant; died of heart failure at 64
Son developed palpitations in his 20s |
| Social/Occupational History: |
Lifelong nonsmoker; does not drink alcohol or caffeine; no illicit drug use
She enjoys the outdoors and recently began participating in triathlons at a recreational level |
Physical Findings
| *Age: |
48 years |
| *Gender: |
Female |
| Race: |
Caucasian |
| *Blood Pressure: |
135/82 mm Hg |
| *Pulse: |
58 bpm |
| General Appearance: |
Comfortable |
| Skin (as necessary): |
Small red-purple nonblanching papules scattered on the trunk |
| Head and Neck: |
Jugular venous pressure 6 cm H2O, normal carotid impulses |
| Chest and Lungs: |
Clear lung fields |
| *Cardiac Exam: |
Regular rate and rhythm, normal S1 and S2, no murmurs at rest or with Valsalva maneuver |
| Extremities (pulse, edema, etc.): |
Peripheral pulses 2+ throughout
Extremities warm, well perfused, no edema |
| Additional Information: |
Blood urea nitrogen of 31 mmol/L, creatinine of 1.7 g/dL
N-terminal pro–brain natriuretic peptide of 1441 pg/mL
Urine microalbumin of 5.1 mg/dL with a microalbumin-to-creatinine ratio of 123.2 mg albumin/g creatinine. |
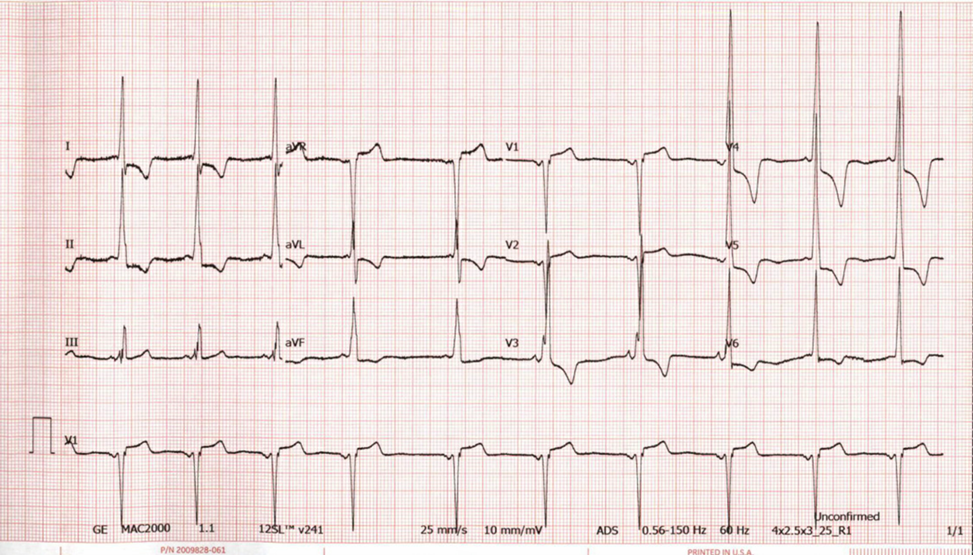
An electrocardiogram (ECG) is notable for sinus rhythm (Figure 1). There is a short P-R interval. There is markedly increased QRS amplitude in the limb and precordial leads with associated repolarization abnormalities.
Figure 1
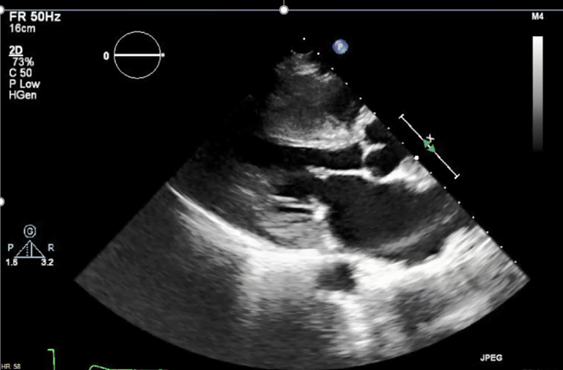
Transthoracic echocardiography reveals left ventricular hypertrophy (LVH) and normal left ventricular (LV) systolic function with an ejection fraction of 65% (Figure 2).
Figure 2
The correct answer is: D. The history and clinical features are most consistent with a multisystem disease phenocopy for hypertrophic cardiomyopathy.
Answer choice D is the correct choice. In constructing the differential diagnosis, it is important to consider key information provided by review of systems and family history, in this case pointing to evidence of multisystem, genetic disease. In this case, one would want to consider multisystem disease that affects the heart, and other affected systems, and that has a genetic basis.
Cardiac manifestations of Fabry disease are an important cause of morbidity and mortality. Over 80% of patients will have cardiac involvement, and a cardiac-predominant variant of Fabry can be difficult to distinguish from HCM. LVH is due to globotriaosylceramide accumulation and interstitial remodeling. Hypertrophy is usually concentric and papillary muscle involvement can be prominent. Systolic function is usually normal. Myocyte vacuolation and myocyte hypertrophy are seen histologically. Myocardial fibrosis develops in the later stages. Cardiac disease increases with age and disease progression, correlating with renal function and alpha-galactosidase A (alpha-Gal A) activity. Atrial arrhythmias, bradyarrhythmias and conduction disease, and ventricular arrhythmias can occur. Progressive heart failure is also seen, typically with normal LV ejection fraction.
Fabry disease is the most prevalent lysosomal storage disorder. It is caused by mutations in the galactosidase alpha (GLA) gene, encoding alpha-Gal A, located on the X chromosome. These mutations lead to absent or deficient alpha-Gal A activity resulting in glycosphingolipid accumulation in lysosomes in different cell types including the kidney, heart, nervous system, vascular endothelium, eyes, and skin. This leads to multisystem disease. The worldwide prevalence is approximately 1:40,000 (~3,000 patients in the United States).
LVH and paroxysmal atrial fibrillation are the key cardiac features of this case. Focusing on the former, when constructing a differential diagnosis, it is helpful to consider five basic paths that result in increased LV wall thickness:
- Secondary LVH due to pressure overload (e.g., hypertension, aortic stenosis)
- Physiologic LVH from intensive athletic conditioning
- Primary myocardial disease/cardiomyopathy
- Infiltrative processes involving the heart
- Prototypically cardiac amyloidosis
- Storage disease involving the heart
- Prototypically Fabry disease
In this case, there is evidence of a multisystem process involving the heart, gastrointestinal tract (chronic diarrhea), skin (papular rash), and kidneys (mild renal insufficiency and microalbuminuria). There is also a signal of familial disease with dominant inheritance over three generations involving the heart (heart failure, atrial fibrillation, conduction disease) and kidneys—again, pointing to a multisystem disorder. There are not enough relatives to determine whether the inheritance pattern is autosomal dominant or X-linked dominant. However, it is important to look for male-to-male transmission. If present, X-linked disorders can be excluded. Constructing a pedigree is an extremely useful visual aid for understanding family history.
A. Answer choice A is an incorrect choice because, although low QRS voltage on ECG is stereotypically described with cardiac amyloidosis, it is not always present and cannot reliably exclude disease.
B. Answer choice B is an incorrect choice because the degree of abnormalities seen on ECG suggests a cardiomyopathic process rather than benign physiologic remodeling. Additionally, the amount of athletic training described (recreational participation in triathlons) in a middle-aged woman is unlikely to result in significant remodeling.
C. Answer choice C is an incorrect choice because the multisystem involvement present in both the patient and her family would be unusual for HCM.
Educational grant support provided by: Bristol Myers Squib
To visit the course page for the Hypertrophic Cardiomyopathy: Accelerating Guideline-Driven Care grant, click here.
References
- Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers 2018;4:38.
- Seydelmann N, Wanner C, Störk S, Ertl G, Weidemann F. Fabry disease and the heart. Best Pract Res Clin Endocrinol Metab 2015;29:195-204.
- Hilz MJ, Arbustini E, Dagna L, et al. Non-specific gastrointestinal features: could it be Fabry disease? Dig Liver Dis 2018;50:429-37.
- Adalsteinsdottir B, Palsson R, Desnick RJ, et al. Fabry disease in families with hypertrophic cardiomyopathy: clinical manifestations in the classic and later-onset phenotypes. Circ Cardiovasc Genet 2017;10:doi: 10.1161/CIRCGENETICS.116.001639.
- Ho CY, Day SM, Ashley EA, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018;138:1387-98.